H5N1 bird flu vaccine, Cytokinetics, Gilead
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Good morning. Wall Street had a big reaction to H5N1 bird flu news yesterday. Was it warranted? We’ll discuss that today.
The need-to-know this morning
U.S. in talks with mRNA vaccine makers on bird flu
While there is no indication the U.S. government plans to order mass production of vaccine for H5N1 bird flu at this point, Dawn O’Connell, assistant secretary for preparedness and response at HHS, said yesterday that some additional steps are being taken.
Nearly 5 million doses of H5 vaccine stored in bulk in the National Pre-Pandemic Influenza Vaccine Stockpile will be put into vials over the next couple of months in case it is needed. Talks are also proceeding with messenger RNA vaccine makers about potentially making batches of H5 vaccine that could be tested and stockpiled; O’Connell said she hopes to have a decision on who the government will work with “very soon.”
STAT’s Helen Branswell reports that Moderna confirmed that it is involved in those negotiations regarding its candidate H5 vaccine, mRNA-1018, which it began testing in a Phase 1/2 trial last summer. The vaccine targets the exact clade of the virus responsible for the outbreak in dairy cattle. “We remain committed to using our mRNA platform to respond to public health concerns,” Moderna said in an emailed statement. The company will not reveal the dosages it is testing, saying it has withheld that information from a public registry of the trial “for competitive reasons.”
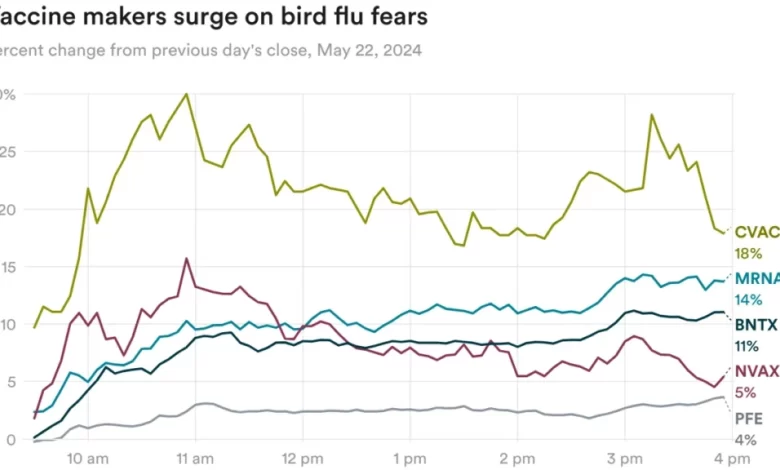
Wall Street, meanwhile, appears to be paying attention. Yesterday, Australia reported its first human case of H5N1 bird flu — in a child who had contracted the virus in India — and Michigan also reported a case, leading shares of vaccine makers CureVac, Moderna, BioNTech, Novovax, and Pfizer to surge.
Are these reactions overblown? Mizuho analyst Jared Holz wrote that he “would expect these storylines to dissipate unless the strains get more powerful, leading to more severe reactions.”
“Would be very surprised, that unless many more cases are reported, that the use of covid-like vaccines will be pervasive enough to greatly alter revenue figures for the likes of MRNA or other players and find the price action as unsustainable,” he said.
A Cytokinetics buyout seems more unlikely now
Cytokinetics said yesterday that it agreed to pay Royalty Pharma a higher royalty on a heart drug that’s expected to launch soon, in return for up to $575 million.
Shares of Cytokinetics sunk in post-market trading as investors viewed the deal as lowering the odds that the biotech will be acquired by a large pharma company. Investors are skeptical that Cytokinetics can independently market its heart drug, called aficamten, as it goes up against a similar treatment sold by pharma giant Bristol Myers Squibb.
Read more from Matt Herper and me.
Gene silencing is latest target for obesity drug developers
ILLUSTRATION BY MARIAN F. MORATINOS
Each quarter, my colleague Allison DeAngelis and I meticulously sift through company releases and clinical trial info to gather all the details about the latest obesity drugs in development.
In the newest update of our obesity drug tracker, we now list roughly 115 treatments in development, a sign of how intense the competition is in obesity. We also noticed an emerging trend — some companies are now attempting to use RNA interference to develop treatments that can have longer-lasting weight loss effects than Wegovy or Zepbound.
Regeneron, Alnylam, Wave, and Arrowhead are running studies on preclinical RNAi candidates, and even Novo Nordisk sees potential in this mechanism.
Read more about the genetic targets these companies are going after, and check out our updated obesity drug tracker. (We’ve added some new features that make it easier to sort drugs by development phase and route of administration.)
Combining GLP-1s with software treatments
Click, a digital therapeutics company, is trying something new with GLP-1 drugs: It’s acquiring the assets of another company called Better Therapeutics, which developed a software-based treatment for diabetes, and will try to adapt the technology for use alongside GLP-1 medications.
Better’s software, called AspyreRx, is a prescription app that delivers cognitive behavioral therapy and had been shown to reduce A1c levels in people with type 2 diabetes. Though this technology was approved by the FDA, it was slow to gain commercial traction, and Click is hoping to revive it by combining it with drugs.
Read more from STAT’s Mario Aguilar.
Gilead’s coronavirus antiviral shows promise in mice
In a new mouse study, a small molecule antiviral from Gilead, called obeldesivir, was found to be effective against a range of coronaviruses, including ones that so far have only been identified in animal populations and could potentially spill over into humans.
Even with effective vaccines, there still remains a need for new coronavirus antivirals to protect people who are immunocompromised or unvaccinated. It remains to be seen how obeldesivir will perform in humans.
Read more from STAT’s Annalisa Merelli.