Skin-inspired, sensory robots for electronic implants
Bio-inspired multi-modal sensory soft robot
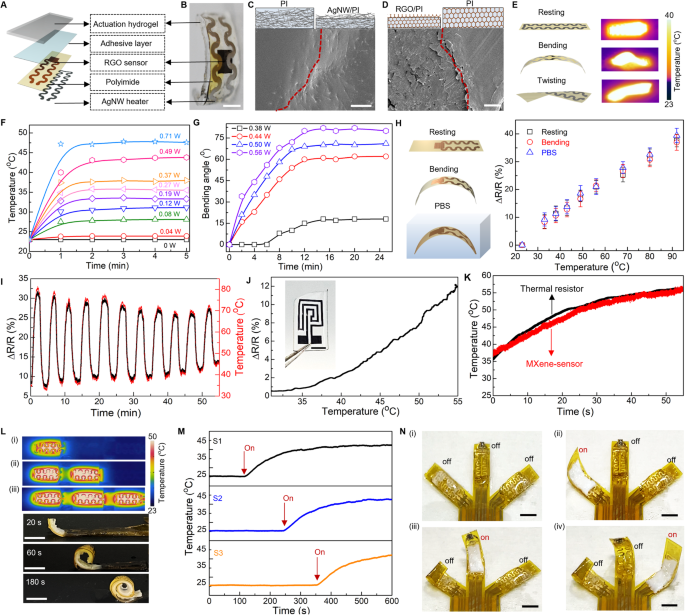
The integrated architecture between the skin and skeletal muscles enables safe and closed-loop interactions with surrounding environment, bringing a feeling of touch and acting of motion seamlessly together in space and time33,34,35. A feature of particular interest in skin is the mechanoreceptors localized at the interface between the epidermis and dermis of skin, which are responsible for detecting a variety of mechanical stimuli, including the fast-adapting (FA) receptors that respond to dynamic forces and slow-adapting (SA) receptors that respond to static pressures (Fig. 2A)36,37,38. By mimicking the hierarchical architectures of skin and muscle with associated biological functions, our soft robots integrate multi-electronic modules and thermally actuatable hydrogels, realizing both receptor-like sensing functions to detect various stimuli, and on-demand muscle-like contraction to generate physically adaptive motion, from a single integrated platform, endowing soft robots with intelligence in navigating through real-world environments autonomously (Fig. 2B). Figure 2C shows a fabricated multi-modal sensory soft robot with geometry emulating a starfish. The robot consists of three primary layers: a flexible nanocomposite layer as a multi-modal electronic-skin (e-skin) embedded with distinct sensors (strain, pressure, pH, and temperature) and stimulators (thermal and electrical), a thermally responsive hydrogel layer as an artificial muscle generating actuation force, and a thin bio-adhesive layer as a cushioning medium to form interfacial binding between the e-skin and artificial muscle. Our approach for fabricating the flexible nanocomposite layer can be generally applicable to a wide variety of soft materials and nanomaterials heterogeneously composited within a single matrix material, which enables the potential to form highly integrated systems with a broad range of sensors and stimulators. Specifically, here we demonstrate (1) a thermal sensor made of a nanocomposite of reduced graphene oxide (RGO) and polyimide (PI), (2) a strain sensor made of a nanocomposite of silver nanowires (AgNWs) and polydimethylsiloxane (PDMS), (3) sensing and stimulation electrodes made of a nanocomposite of poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT:PSS) and PI, and (4) a thermal heater made of a nanocomposite of AgNWs and PI. The flexibility and versatility of this strategy allow the as-fabricated functional units to be easily integrated into each arm of the starfish robot in a monolithic fashion, as illustrated in Fig. 2D, realizing a sensory skin for the robot to enable environmental awareness. Figure 2E displays an example of an ultrathin multi-modal e-skin equipped with six nanocomposite sensors (The detailed fabrication process appears in Fig. S2A.). The e-skin is further encapsulated with a parylene layer (thickness ~2 μm) to enhance its durability during prolonged applications39,40. This is subsequently attached onto a piece of predesigned thermally responsive PNIPAM hydrogel that serves as an artificial muscle for the soft robot (Fig. S2B). The PNIPAM hydrogel can undergo a dramatic volumetric reduction of about 90% as the temperature shifts from 25 °C to 60 °C. Notably, this significant and rapid deswelling behavior is initiated only when temperature is beyond its lower critical solution temperature (LCST 32–34 °C), enabling versatile actuation capabilities within biological environments (Fig. S3A, B, and Fig. 2H)41,42. Additionally, we can adjust the LCST by incorporating acrylamide (AAm) into the PNIPAM hydrogel to form poly(NIPAM-co-acrylamide) (P(NIPAM-AAM)), enabling us to tailor the LCST to align with varying application scenarios that may require operations at distinct temperature ranges (Fig. S4)43,44,45. The as-fabricated soft robot can actively form a conformal and stress-free interface with curvilinear biological surfaces, indicating its inherent mechanical softness and high biocompatibility. This adaptability minimizes potential risks related to mechanical incompatibility, facilitating its smooth integration with targeted tissues/organs (Fig. 2F, G, and Fig. S3C). At an elevated temperature (40 °C), the robot consisting of the stimuli-responsive artificial muscle (PNIPAM layer) and non-stimuli-responsive e-skin (multi-modal layer) tends to bend toward the muscle side on the basis of asymmetrical responsive properties (Fig. 2H, Fig. S5A–C)17,46.
A Schematic illustration of epidermis-dermis-muscle structure of skin. B Bio-inspired structure of soft robot from skin. C Conceptual illustration of the integrated multi-modal sensory soft robot with distinct nanocomposite sensors functionalized into each arm. D Schematic illustration of a starfish-inspired multi-modal sensory soft robot. Left: An exploded view highlighting 3 primary constituent layers, including a flexible multi-modal layer, a bio-adhesive layer, and an actuation hydrogel layer. Right: Schematic illustration highlighting the multi-material integration within the multi-modal layer including: (i) Silver nanowires (AgNWs) and polyimide (PI) as a flexible heater; (ii) AgNWs and PDMS as a strain sensor; (iii) Reduced graphene oxide (RGO) and PI as a temperature sensor; (iv) Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) and PI as sensing and stimulation electrodes. E Optical image of the flexible e-skin with six nanocomposite sensors. Optical image showing conformal attachment of the soft sensory robot onto human skin (F) and porcine tissue (G) with high mechanical compliance. H Top: Schematic showing a temperature-responsive bending from a bilayer of poly(N-isopropylacrylamide) (PNIPAM) and polyimide-based nanocomposite. Bottom: Volumetric shrinkage of PNIPAM across a temperature range of 25–60 °C. The data points represent the mean value from n = 3 independent experiments, and the error bars are in S.D. I–K Optical images and corresponding finite element modeling of thermal-triggered structural reconfiguration of soft robots. Colors indicate von Mises stress magnitude. I Biomimicry soft gripper encloses upon heating at 40 °C. J Chiral seedpod-inspired robot reverses helix at 40 °C. K Soft robotic pill expands upon heating at 40 °C. L, M Schematic and optical images of anisotropic integration of various functional materials into a polymeric matrix to form a multi-modal sensing system using an in situ solution-based approach. L A RGO/PI-based temperature sensor and an AgNW/PI-based heater on the same polyimide side. M PEDOT:PSS/PI-based electrodes and RGO/PI temperature sensors integrated on the two opposite sides of a PI layer, respectively. Scale bars, 5 mm.
Figure 2I, Fig. S5D, and Supplementary Movie S1 provide an example of a robotic starfish reversibly closing and opening its rays under a temperature shift between 23 °C and 40 °C. Furthermore, such multi-layer integration allows a diverse collection of robotic designs that undergo various types of actuations. Fig. S6, 7 and Supplementary Movie S2 highlight soft sensory robots featuring starfish-like structures with an arbitrary number of rays (e.g., four and six) and a fishbone-like structure with four pins. In addition, tuning the design layout of the multi-layer structure can yield complex deformation and structural reconfiguration beyond simple bending motion. Here, inspired by the self-twisting of chiral seedpods, we constructed a geometry with parallel strips in the e-skin layer (Additional details appear in Fig. S8A)47,48. Upon thermal stimulation, the PNIPAM hydrogel contracts while the PI maintains stability, generating a differential contraction across the structure. The differential thermal response induces a tilting torque, leading to local saddle-like curvature and twisting motion of the integrated robotic systems (Fig. 2J, Fig. S8B and Supplementary Movie S3). Our findings closely align with the simulation results as shown in Fig. S8C. We also develop a soft robotic pill based on an anchored hydrogel/nanocomposite tri-layer structure, where a hydrogel-muscle layer is surrounded by two separate e-skin layers that bond to the muscle layer at the edges (Fig. S9A shows the detailed structure design.). Upon thermal actuation, the pill can self-expand into a 3D ring shape, since the contraction of the muscle layer drives the e-skin layers to buckle out of the plane, as shown in Fig. 2K, Fig. S9B, and Supplementary Movie S4. Furthermore, compared with a make-and-transfer method30,31,32, our in situ solution-based fabrication approach enables the integration of sensors into the e-skin matrix in a single step, enhancing mechanical and electrical performance by reducing interfacial resistances and improving mechanical conformity, thereby significantly improving sensitivity and responsiveness. As aforementioned, this approach also offers a versatile platform that can be constructed using a broad range of functional nanomaterials hybridized with a polymeric matrix to form a multi-modal sensing system. By selecting materials that offer unique functional attributes, from AgNWs known for their conductivity and flexibility to graphene and MXene for their high surface area, electrical conductivity, and hydrophilicity49,50,51,52, this system provides a possible emulation to the skin that contains complex somatosensory units, where various mechanoreceptors and thermoreceptors distributed in the epidermal and dermal layers enable the spatiotemporal recognition of the magnitude and location of touch and temperature stimuli53,54. Fig. S10 provides a representative example of in situ integration of AgNW/PDMS-based strain sensor, in which the strain sensor has a good response to the stretching. Figure 2L shows the proposed solution-based approach enables a freestanding PI film to integrate multi-functional modalities including an RGO/PI-based temperature sensor and an AgNW/PI-based heater (Fig. S11A), endowing soft robots with both thermal sensing and stimulation. Moreover, Fig. 2M displays a more complicated integration paradigm with multi-layer stacking, where different electronic components (e.g., PEDOT:PSS/PI-based conductive electrodes and RGO/PI temperature sensors) can be distributed in different layers of the e-skin to achieve simultaneous functional versatility and compactness. This assembly technique ensures the e-skin remarkable thinness and flexibility, enhancing its effective performance for implantable applications (Fig. S11B). The X-ray photoelectron spectroscopy (XPS) characterization on the e-skin layers reveals the precise nanoscale integration of active materials within a polymer matrix, as detailed in Figs. S12–S14 and Supplementary Note S1. It showcases the optimal distribution and intermolecular bonding of the composite components, effectively addressing the common challenge of uneven dispersion of nanomaterials, which usually undermines the performance of conventional composites. Our approach minimizes the polymer amount required to integrate nanomaterials into composite functional modules and utilizes excess polymer as an insulating layer to separate modules, preventing interference between their electrical and chemical signals, thereby ensuring that each functional module operates independently and effectively. This simple approach combines the distinct properties of each constituent, achieving a balance between structural integrity and functional versatility55,56,57,58,59. This advanced level of integration would be of great value for soft robots that seek to achieve multifunctionality and local sensing capabilities approaching skin.
On-demand robotic actuation with spatiotemporal control
Programmable stimuli-responsive soft robotic systems capable of working in enclosed or confined spaces and adapting functions under changing situations hold great promise as next-generation medical robots60,61. The realization of versatile morphing modes through local-actuation control is crucial for enhancing on-demand actuation. Here, we develop soft robots with cognitive capabilities via unifying programmable actuation and in situ sensing. Figure 3A shows a sensory robotic arm primarily consisting of a PNIPAM-hydrogel-based muscle layer and a multi-modal e-skin layer that is designed with an AgNW/PI nanocomposite actuation heater and a RGO/PI nanocomposite thermal sensor. Fig. S15A shows layer-by-layer stacking as a simple and effective approach for fabricating the e-skin. This approach stands out for its capability to fabricate multi-layered e-skin integrating diverse functionalities within an unified e-skin framework, offering a sophisticated level of device customization. Figure 3B and Fig. S15B demonstrate that the multifunctional nanocomposite film is highly flexible and can be tightly bonded onto the hydrogel layer via n-butyl cyanoacrylate adhesive. Importantly, the bio-adhesive layer exhibits robust adhesion under both dry and wet conditions, as displayed in Fig. S16. This feature ensures the reliability of implantable devices in the dynamic and moist human body environment. When the liquid PI is cast onto the nanomaterial film (e.g., AgNWs and RGO), the liquid PI penetrates into the interconnected pores of the three-dimensional (3D) network, owing to the low viscosity and low surface energy of the liquid PI62. The scanning electron microscope (SEM) images, including both top and side views as shown in Fig. 3C, D, Fig. S17A–F, and Fig. S21A–C, present that the curing process fully buries all the nanomaterials inside the PI matrix, resulting in a uniform composite free from observable voids. This prevents separation of the nanomaterials from the polymeric matrix, thus minimizing untended side effects to the neighboring tissues. The Fourier-transform infrared spectroscopy (FTIR) and X-ray powder diffraction (XRD) results (Fig. S17G, H, Fig. S21D, E, and Supplementary Note S2) also confirm the chemical structure of AgNW/PI and RGO/PI nanocomposites suggesting a successful fabrication of high-quality nanocomposites of functional materials and polymer matrix63,64,65,66. Figure 3E demonstrates that the resultant AgNW/PI nanocomposite conductor is highly conductive, twistable, and bendable, and thus used as a highly flexible heater for electrothermal actuation. Thermal images in Fig. 3E show the infrared thermograph of a Joule-heated e-skin in the resting state and under both bending and twisting conditions. The electrical heater distributed inside the e-skin exhibits a stable and uniform temperature distribution without degradation of the temperature level at the deformed points, ensuring effective thermal transport from the heater to the hydrogel-based muscle layer. Figure 3F and Fig. S18A depict the transient electrothermal response of the flexible heater applied by various powers in an ex vivo environment. The saturation temperature of AgNW/PI nanocomposite heater increases with the supplied power as more Joule heat is generated, and the lower critical solution temperature (LCST) of PNIPAM hydrogel at 34 °C can be obtained at low input power (<~1 W) (Fig. S18A). As shown in Fig. S18B, the flexible electrical heater exhibits steady heating and cooling cycles, indicating high repeatability and remarkable heating stability using AgNW/PI nanocomposite. Figure 3G shows varying the amount of electric power applied to the heater effectively modulates the resultant bending angle θ (as defined in Fig. S19A) of the robotic arm, thus realizing precise, on-demand access to intermediate morphologies along the bending pathway. Figure 3G also indicates that the actuator reaches its peak deformation upon achieving thermal equilibrium, and importantly, this maximum bend is maintained as long as there are no changes in thermal conditions. We further evaluated the mechanical force generated by the soft robotic finger which incorporates a PNIPAM hydrogel layer roughly 1 mm thick, under various input powers. Fig. S19B shows that the static force exhibits a noticeable increase with rising temperature. At a temperature of 40 °C, the force reaches a maximum of 32 mN. Additionally, it is observed that the generated force remains consistent throughout 40 cycles of alternating power on and off (0.35 W), indicating the robust reversibility of the soft robot (Fig. S19C). When compared to similar hydrogel-based soft actuators, our design consistently achieves a relatively high output force, as shown in Table S167,68,69,70,71,72,73,74. Moreover, our study demonstrates that the thickness of the hydrogel layer determines the actuation performance of the device. As depicted in Fig. S20A, thicker hydrogel layers induce more pronounced shape morphing at a set activation temperature, while a slower response due to their increased mass. The thicker layers are also capable of generating higher actuation force, thereby enhancing the actuation capability of the device (Fig. S20B). However, the increased device volume may limit its applicability in minimally invasive implantable devices, where compactness is a key factor. In the device configuration, embedded sensors are strategically positioned within a single, thin e-skin layer, thus minimizing the required thickness of the hydrogel layer for efficient actuation and allowing the integrated system to be relative compact (Fig. S20C). Our fabrication technique accommodates customization of the hydrogel layer thickness to optimize device performance for specific applications, demonstrating the method’s flexibility and adaptability to various biomedical needs. The utilization of a RGO/PI-nanocomposite temperature sensor enables real-time tracking of environmental temperature during muscle motion. The in situ solution processing ensures RGO network is uniformly distributed in PI matrix to provide precise pattern registration with good electrical conductivity (~400 S/m) and stable performance in aspects of electrical linearity, sensitivity, and repeatability. Figure 3H illustrates the resistive change in a relatively linear relation with temperature for the RGO/PI thermal sensor. The temperature coefficient of the resistance (TCR) of the RGO/PI thermal sensor is >0.5%/°C, featuring its high thermal sensitivity. On the other hand, the RGO/PI-based thermal sensor exhibits a stable performance after 1000 bending cycles, and even after immersing in PBS solution. Figure 3I and Fig. S21F show performance of the thermal sensor in response to cycles of temperature rise and drop, indicating good sensing stability. In addition, Fig. S21G shows consistent measurements of RGO/PI nanocomposite sensing performance in comparison with a commercial thermal resistor (ERT-J0ET102H), indicating excellent sensing accuracy. To exemplify this versatility, we have also successfully fabricated MXene-based sensors, as illustrated in Fig. 3J, K and Fig. S22. MXene-based materials possess inherent biocompatibility, rendering them ideally suited for incorporation into implantable biomedical devices without eliciting adverse reactions or compromising the surrounding biological environment52,75.
Illustration of an exploded view (A) and optical image (B) of a soft robotic arm comprising a PNIPAM hydrogel layer, a RGO/PI thermal sensor, and an AgNW/PI actuation heater, bonded using n-butyl cyanoacrylate adhesive. Joule heating from the AgNW/PI heater triggers bending of the robotic arm. Scale bar, 5 mm. C, D SEM images of nanocomposite film surfaces. Here, deep reactive ion etching (DRIE) of partial regions reveals the anisotropic integration within the films, distinguishing pristine PI from AgNW/PI (C) and RGO/PI regions (D). AgNWs and RGO, with higher surface-free energy than PI, are uniformly dispersed inside the PI matrix, enhancing wetting and binding properties. Scale bars, (C) 1.5 μm, (D) 10 μm. E Infrared thermograph of an AgNW/PI heater demonstrating consistent heating performance even after 1000 bending and twisting cycles. F Surface temperature of this heater varies with input electric power, functioning efficiently at low power. G The bending angle of a soft robotic arm changes with varying input electric power. H Resistive response of the RGO/PI thermal sensor during bending, twisting, and PBS immersion, across temperatures from 23 °C to 92 °C. Data points represent mean values from n = 3 independent experiments, error bars in S.D. I Static cycling test of the RGO/PI thermal sensor. Here, the left y-axis represents resistance changes, while the right y-axis shows corresponding temperature changes. J Resistive response of an MXene-based thermal sensor across temperatures from 23 °C to 55 °C. K Temperature measurement comparison between the MXene/PI thermal sensor and a commercial thermal resistor (ERT-J0ET102H). L Stepwise coiling actuation of a soft robotic probe via sequential thermal stimulus. Top shows infrared thermograph and bottom shows structural changes from a flat to coiled state. Scale bars, 10 mm. M Integrated RGO/PI thermal sensors enable temperature measurement of localized regions for proprioceptive sensing. N Optical images demonstrating on-demand motion control of a three-arm soft robotic gripper via sequentially programming input power. Scale bars, 5 mm. 3 measurements are repeated with similar results to (C), (D), (I), (J), (K), (L).
Well-controlled shape morphability with high spatiotemporal resolution is essential for soft robots to execute complicated tasks safely in biological environments76,77. Here, the integration of e-skin and artificial muscle allows the bio-inspired soft robots to realize motions of local regions independently, which collectively can lead to a wide range of shape morphability. Figure 3L demonstrates the locally controlled morphability with a soft robotic finger, of which the e-skin includes a series of AgNW/PI-based heaters for localized thermal activation, and RGO/PI-based thermal sensors for temperature monitoring (Fig. S23A). As shown in Fig. 3L and Fig. S23B, the robotic finger undergoes a stepwise self-coiling motion via a sequential stimulus and perceives its local temperature simultaneously by the embedded thermal sensors (Fig. 3M). More complex actuation modes or configurations are accessible owing to the combined ease of processing and ability to introduce multifunctional units. Figure 3N presents a three-arm soft robotic gripper where each arm contains various types of sensors, including an optical sensor, a thermal sensor, and a strain sensor, respectively, and an electrical heater (Fig. S24A), to enable independent control of motion for each arm, thus allowing on-demand gripping and interaction with targeted objects (Supplementary Movie S5). Here, the observed variability in bending configuration can be attributed to differences in the positioning and depth of the functional modules within the e-skin layer. Moreover, the soft robotic systems can be structurally tailored to support a range of motions. For example, Fig. S24B, C highlights a soft starfish-like robot with four sensory arms, and a soft robotic cuff with optoelectronic sensors, respectively. The electrothermal stimulus along with distributed sensing capabilities enables programmed actuation not only on demand but also regulated simultaneously by the sensing feedback (e.g., Supplementary Movie S6).
Furthermore, our soft robotic system exemplifies advanced sensory-motor integration, leveraging the synergistic relationship between embedded sensors and actuators to achieve dynamic adaptivity and responsiveness to environmental changes. A prime example is a temperature-sensitive control system, as shown in Fig. S25A, which utilizes real-time sensory feedback to dynamically adjust heating in response to environmental temperature changes. The operational principle, as detailed in Fig. S25B and Supplementary Note S3, involves a microcontroller-driven algorithm that interprets temperature input collected by a resistive temperature sensor, and modulates the electric heater’s current accordingly, enabling rapid adaptations to achieve and maintain a preset temperature. Fig. S26 presents a soft robotic finger’s real-time response to temperature variations, ensuring stable shape adaptation through this regulatory mechanism. Moreover, this intelligent control significantly improves safety by preventing the risk of overheating, thereby ensuring the system’s safe operation in various thermal conditions, highlighting our device’s ability to provide precise thermal management, enhancing both efficacy and safety in its applications78,79.
Wireless sensing and actuation of soft sensory robot
Wireless operation of actuation as well as therapeutic and/or diagnostic functions is essential for implantable robots to minimize tissue damage and implant infection76,80,81,82. However, existing schemes for wireless operation of robots mostly require sophisticated circuitry for energy harvesting and storage, which may introduce non-negligible heat (~80 °C), unfavored space occupation, system complexity, limited lifetimes, and high cost83,84. To overcome the preceding challenges of actuation/sensing integration in soft implantable robots, we report a bio-inspired soft robot as a representative example to demonstrate remote, battery-free operation and communication in both sensing and actuation.
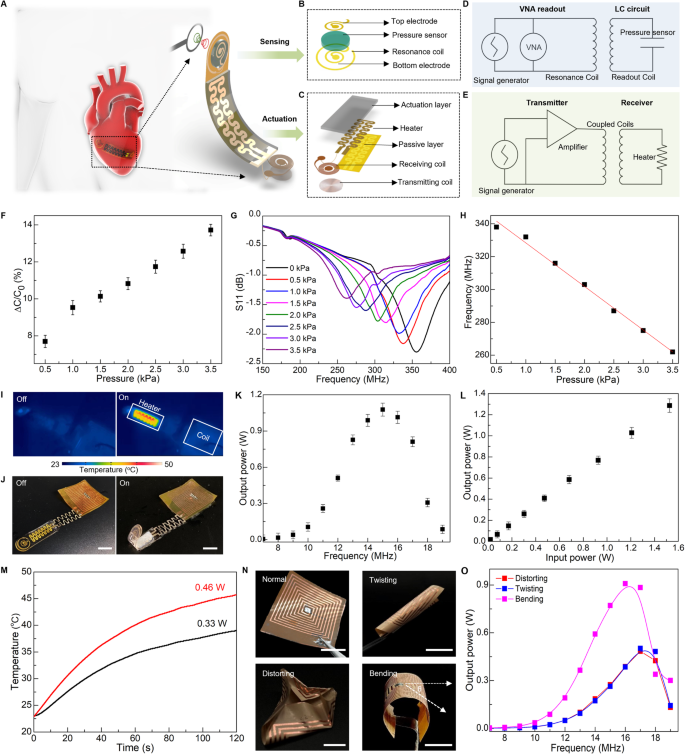
Figure 4A–C show schematic illustrations of the system design consisting of sensor and actuator components. Our strategy for wireless sensing relies on a passive inductor-capacitor (LC) resonance circuit formed by a planar inductor coil and a parallel plate based on capacitor polyacrylamide (PAAm) hydrogel, as illustrated in Fig. 4B. A change in a biomechanical event (e.g., vessel filling, cardiac contraction/relaxion, and bladder filling/voiding) can lead to a corresponding change in capacitance of the hydrogel-based capacitor, which can be quantified by recording shift in resonance frequency (fs) of the LC circuit according to the equation \({f}_{s}=1/2\pi \sqrt{{LC}}\), where L and C are the inductance and capacitance of the resonance circuit, respectively85,86. The inductor coil couples to an alternating electromagnetic field through a readout probe, enabling quantitative measurement of the input return loss (S11) using a vector network analyzer (VNA) (Fig. 4D and Supplementary Note S4). The proposed PAAm-based sensor with relatively low modulus, intrinsic stretchability, and biocompatibility, can detect the pressure through variation of capacitance between the two electrodes. Figure 4F shows a linear correlation between the measured ΔC/C0 and applied pressure. The slope of the linear fitting curve reports gauge factor (GF) of the sensor reaching ~3%/kPa in the clinically relevant range of pressure (0–4 kPa). Figure 4G&H shows the resonant frequency decreases from 340 MHz to 260 MHz in response to the applied pressure decreasing from 0 to 3.5 kPa, and the pressure sensitivity of the sensor is ~26.7 kHz/kPa.
A Schematic of a soft sensory robot featuring an electrical heater, a polyacrylamide (PAAm)-based pressure sensor, and two inductive coils for transmission sensing signals (B) and electrical power (C). B Exploded view of the sensing components containing a capacitor with two electrodes, a PAAm-hydrogel dielectric layer, and a copper (Cu) inductive communication coil. C Exploded view of actuation components consisting of a PNIPAM actuation hydrogel, a flexible electrical heater, and a radiofrequency (RF) power harvester based on a copper coil. D Equivalent circuit diagram of wireless pressure sensing, where pressure variations alter the capacitance and, thus, resonance frequency which is captured wirelessly through a vector network analyzer (VNA). E Equivalent circuit diagram of wireless actuation, where a transmitting coil connected to an RF power amplifier energizes the receiving coil, powering the heater for robotic motion. F Measured capacitive change of the PAAm-based pressure sensor in response to applied pressure. G Measured shift of resonance curves of the PAAm-based pressure sensor in response to applied pressure. H Change of the LC resonant frequency as a function of applied pressure serving as a signal-transduction scheme for wireless pressure detection. I Thermal distribution during wirelessly harvesting energy exhibits minimal heating in the receiving coil and efficient power consumed by the electrical heater, ensuring minimal heat damage to surrounding bio-environments. J Optical images of a soft sensory robot undergoing a wireless actuation to transform from a flat state to a bent state. K The output power as a function of frequency, optimized at ~15 MHz. L The output electrical power varies with the input power. M The temperature change of the electrical heater over time under various output powers used for wireless actuation. N Optical images of the deformed RF coils including bending, twisting, and distorting. O Measured resonance frequency of RF coil under various shape deformations. Here the bending angle θ is 60o. Scale bars, 5 mm. Data are presented (F), (K), (L) from n = 3 independent experiments, and the error bars are in S.D.
Moreover, wireless electromagnetic power transmission is an attractive solution to overcome limitations imposed by implantable systems with discrete batteries, especially for devices operating in enclosed places such as the human body86,87,88. Here, we demonstrate the bio-inspired, sensory robot allows for electrically controlled locomotion in this wireless, battery-free manner (Fig. 4A). Figure 4C shows layout of the wireless actuation design including a PNIPAM actuation hydrogel, a flexible electrical heater, and a radio-frequency (RF) power harvester based on a Cu coil. The RF harvester is made of a triple-layer structure (Fig. S27A–C). Magnetic coupling between the transmitting and receiving coil generates electric currents to the heater that thermally stimulates the artificial muscle (Fig. 4E & Fig. S27D). As shown in Fig. 4J and Fig. S28, the robotic finger can undergo a bending motion upon receipt of electromagnetic energy from a power-transfer module. Figure 4I shows an infrared image of the robotic finger highlighting concentrated heat on the electrical heater and minimum heat on the receiving inductor, which collectively ensures a stable power supply and minimum heat damage to surrounding bio-environments in potential usage as implants. Figure 4K shows the output power as a function of frequency. The wireless power transfer system achieves the highest harvested power ~1.05 W at a frequency of 15 MHz. Figure 4L indicates the harvested power can be tuned via varying the input power at the optimal frequency 15 MHz, and the power efficiency reaches around 80%, which is sufficient to trigger the heater for raising local temperature to drive motions of the soft robot (Fig. 4M).
The soft robotic system features spatiotemporal locomotion wirelessly controlled by RF signal modulation. To demonstrate the working principle of this method, we fabricate a soft robotic gripper with three arms as an illustrative example. This strategy employs a three-lead LC receiver coil designed based on the frequency-response characteristics of magnetic resonance coupling (Fig. S29A, B), allowing for the formation of two different configurations with distinct inductive values. These configurations are paired with corresponding capacitors to form LC circuits with different resonant frequencies (Fig. S29C). By coupling with different transmission coils operating at distinct transmission frequencies, the coils can selectively deliver power to specific heaters, thereby enabling the activation of individual robotic arms (Fig. S29D–F). Furthermore, we conduct a further investigation into the influence of shape deformation, including bending, twisting, and distorting, on the transfer performance of the RF harvester (Fig. 4N). Fig. S27E demonstrates that the zeros of the reactance of the receiving circuit remain constant throughout the entire process, indicating minimal disruption to the coil’s resonance frequency89,90. Additionally, Fig. 4K and Fig. 4O exhibit a reduction in transmission efficiency resulting from decreased mutual inductance between the transmission and the receiving coils due to shape deformation. For all the deformations shown here, the resonance frequency of the coil remains within an acceptable range for power harvesting, and the power transmitted remains above the minimum level required for the robotic actuation. Supplementary Note S5 and Figs. S30–S32 further demonstrate the effect of various design and operational parameters on the power transfer system91,92,93. This yields systematic metrics for designing a well-tuned WPT system, capable of fulfilling the specifications of the targeted application, concurrently decreasing power expenditure, and evading possible risks to living organisms78,94. Such wireless robotic systems may serve as a promising solution to safe, real-time monitoring of internal pressure needed for various medical procedures.
Soft sensory robots interfacing with various internal organs
Soft sensory robots have significant potential for medical-device applications that warrant safe, synergistic interaction with humans95,96. Here, we develop robots with the capability to actively morph into 3D configurations to generate a stress-free and stable interface with targeted organs for enhanced sensing, stimulation, and drug delivery. In vitro tests with artificial organ models demonstrate the versatilities and potential applications of our soft robots.
Urinary bladder dysfunction is one of the emerging issues in an aging society, which not only leads to loss of voluntary control over the bladder muscles, but also cuts off sensorial feedback to central nervous system97. In most cases of bladder dysfunction, the patients are not able to sense the fullness of bladder with urination, making it a challenge to time the action for voiding treatment (e.g., electrical stimulation)98. Therefore, realizing the voiding treatment in a timely manner requires a monitoring system that measures the bladder status continuously.
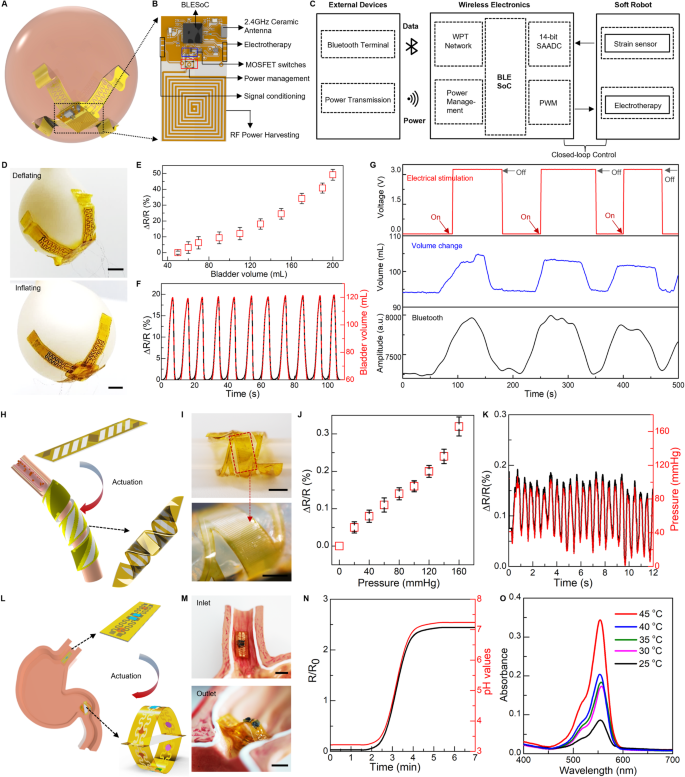
Here, we develop a soft robotic gripper for both real-time assessment of bladder volume and voiding treatment in a wireless closed-loop control fashion. The as-prepared robotic system, illustrated in Fig. 5A–C and Fig. S33A, B, consists of a flexible hydrogel-based actuator, a 3D buckled strain sensor, an electrical stimulator, and a control module. The actuator includes a passive layer made of a patterned Au/PI bilayer as an electrical heater, and an active layer of PNIPAM hydrogel. Upon an electrical trigger delivered via an inductive coil, the robotic gripper can bend and wrap around the bladder conformally and gently (Fig. 5A) to ensure precise measurement of bladder volume and minimize stress at the interface. The strain sensor integrated into the robot can detect bladder volume continuously. Here, the strain sensor includes an elastic PAAm hydrogel film and a serpentine Au/PI resistor to form a buckled 3D structure for enhanced sensitivity. The fabrication steps for the buckled sensor appear in Materials and Methods. We use a balloon model to mimic the natural bladder behavior of filling and emptying to validate performance of the integrated soft robotic gripper. Fig. S33C shows the sensor conformally attaches onto the balloon surface with the biocompatible adhesiveness of PAAm hydrogel99. The softness of PAAm hydrogel in the robot achieves minimal strain disruption to the bladder during the device operation. Injecting and extracting water into the balloon with a syringe pump realizes the behavior of bladder filling and emptying (Fig. 5D). The change of sensor resistance exhibits a strong linear correlation with bladder volume during its expansion and shrinkage (Fig. 5E and Fig. S33D, E), thus serving as an indicator in bladder-volume control. Figure 5F demonstrates the system repeatability in real-time monitoring during multiple cycles of bladder filling and emptying.
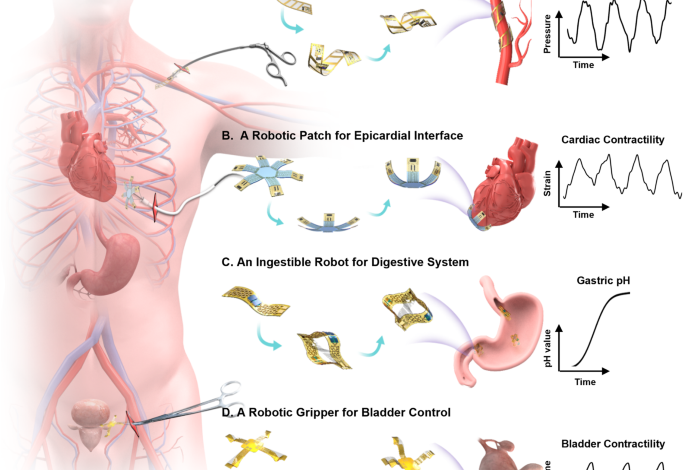
A A fully implantable, soft robotic gripper precisely measuring bladder volume and providing electrical stimulation through wireless closed-loop control. B The control platform including a wireless power harvesting network, a full bridge amplifier, a voltage regulator, and signal conditioning circuits integrated with a Bluetooth System-on-Chip, and a MOSFET switch. C Block diagram for the bladder stimulation module. D A soft robotic gripper deployed onto an artificial bladder, demonstrating deflation and inflation cycles. E Measured resistive characteristics of the buckled strain sensor on the artificial bladder correlated with volume changes. F The 3D buckling strain sensor monitoring real-time volumetric changes during cyclic bladder operations, with resistance and volume changes displayed on dual axes. G Programmed electrical stimulation (top) and measured volume of an artificial bladder (middle and bottom). This demonstration is conducted using a volume threshold of ~100 mL, and amplitude of 3 V. A slight delay in the deactivation process could be attributed to the microcontroller unit’s response time. H A soft robotic cuff enclosing around a blood vessel for monitoring blood pressure. I Optical image of the cuff on an artificial vessel stimulating blood circulation. J Measured resistive changes of the strain sensor at various simulated blood pressures. K Fluidic pressure measurement in an artificial artery system using the soft robotic cuff, displaying resistance changes on the left y-axis and pressure changes on the right y-axis. L A soft ingestible robot designed for continuous stomach pH monitoring and extended drug delivery. M Optical images showing the robot entering, expanding and blocking in the stomach. N Electrical response of PEDOT:PSS/PVA hydrogel to pH change ranging from 3 to 7 over time, with resistance on the left y-axis and pH changes on the right y-axis. O Rhodamine-B embedded into the poly lactic-co-glycolic acid (PLGA) matrix to form a drug delivery patch concealed inside the robot. Its release is measured by UV-vis absorbance over an hour at different temperatures. Scale bars, 5 mm. Data are presented (E), (J) from n = 3 independent experiments, and the error bars are in S.D.
The readout of the bladder volume is achieved through a voltage divider circuit (VDC) consisting of a reference resistor connected in series with the as-fabricated strain sensor. This configuration has minimal impact on the wireless power transmission between coils (Fig. S34). To achieve a closed-loop control of electrical stimulation in the treatment of a dysfunctional bladder system, we further program the Bluetooth-Low-Energy (BLE) System-on-Chip (SoC) (BLE SoC) to enable a pulse-width modulation (PWM) instance. The instance, together with on-board power amplification and filtering circuits, facilitates the application of programmable electrical stimulation in response to an increased strain in the bladder. This enables on-demand electrotherapy and closed-loop control of the robotic implant, as illustrated in Fig. 5B, C. A demonstration of the control scheme can be found in Fig. S35 and Fig. 5G. When the balloon’s volume reaches a predetermined threshold, set here at 100 mL, the control system initiates electrical stimulation. After successful voiding below the threshold, the system automatically deactivates the stimulation. While electrical stimulation has shown promising results in enhancing bladder control in various studies and clinical trials, its efficacy can differ across individuals100,101. The effectiveness of electrical stimulation for bladder voiding and its required voltage levels requires further investigation beyond the scope of our current study. However, our prototype showcases the potential of integrating sensing and actuation mechanisms to facilitate timely and adaptive interventions for bladder dysfunction.
The on-demand motion provided by the soft robot facilitates device implantation and ensures benign and stable contact with targeted tissue or organs12,102,103. Here we develop a soft robotic cuff that can enclose around a blood vessel upon thermal stimulation (Fig. 5H), to enable real-time measurement of blood pressure. As shown in Fig. S36A, the soft robotic cuff consists of a muscle layer based on a PNIPAM and an e-skin layer embedded with a strain sensor based on a serpentine Au ribbon. Notably, the e-skin layer uses a pattern of parallel strips in the PI film, that forms a 45o angle with the longitudinal direction of the device, which, by coupling with the muscle layers, facilitates formation of a helix structure. An in vitro model of artery that uses a rubbery tube with a pulsatile flow of water to create a simulated pulsation pattern validates performance of the soft robotic cuff (Fig. S36B–D and Fig. 5I). The helix formation of the robotic cuff provides a gentle and stable coupling with the artificial artery, of which the measured signal (resistive change) exhibits a linear relation with the inner fluid pressure (Fig. 5J). Figure 5K demonstrates the capability in continuous measurement that captures patterns of simulated pulsation, further confirming its utility in measuring blood pressure.
Furthermore, such bio-inspired designs of soft sensory robots can also configure into an ingestible platform. Here, we show that a soft ingestible robot is capable of prolonged residence in the stomach for pH monitoring and drug delivery. Fig. S37A depicts the proposed structure of the ingestible robot based on a slab-shaped muscle layer sandwiched by two e-skin layers consisting of a drug delivery module and a pH sensing module (Fig. 5L, more details appear in the Materials and Methods.). The miniaturized size of the robot facilitates swallowing and transport through the esophagus to stomach (Fig. 5L). Once in the stomach, the robot self-expands that prevents passage through the pylorus for an extended duration in the gastric environment (Fig. 5M and Fig. S37B). The soft pH sensors based on PEDOT:PSS/poly(vinyl alcohol) (PVA) embedded onto the e-skin of the robot provide continuous measurements of gastric pH (Fig. S37C and Fig. 5N). The pH sensor exhibits a linear relationship between the resistive change and pH of the fluidic environment within the range of acidic (pH ~ 3) to basic (pH ~ 8) (Fig. S37E). To realize prolonged drug release, the integrated drug patches use poly(lactic-co-glycolic acid) (PLGA) as a matrix to load drugs and the robotic motion as a trigger mechanism that exposes the drug-loaded patches to stomach fluid for initiating drug release. As a demonstration, we use a biocompatible dye, rhodamine-B (RB), as a model drug (5 mg of RB per 0.5 g PLGA). Figure 5O and Fig. S37H show the UV-vis absorption spectra and the corresponding dosages, respectively, of the RB-loaded drug patch, immersed in PBS solution (at pH ~ 5) for 1 h under various temperatures ranging from 25 °C to 45 °C, (Fig. S37F, G show the calibration curve based on the measured UV-vis absorption spectra.). Such ingestible soft robots highlight a synergistic combination between sensing function and robotic motion to realize on-demand, prolonged control of drug delivery.
Additionally, our device offers versatile adaptability for diverse application scenarios through customizable dimensions, sensor positioning, and geometric layouts, ensuring it aligns with the unique morphologies and functional demands of targeted tissues/organs. This flexibility is essential for enabling minimally invasive deployment. As illustrated in Figs. S38, S39, Table S2, and Supplementary Note S6, the design’s adaptability enhances soft robotic technologies for effective integration in a broad spectrum of biomedical applications104,105,106,107,108,109,110,111. We also explored the bioadhesive behavior of our device on targeted tissues/organs. We observed that hydrogel’s inherent adhesiveness is significantly related to its water content and temperature112. As shown in Fig. S40, there is a decline in adhesive strength as temperatures approach the hydrogel’s LCST. While this inherent adhesive capability contributes to the initial secure placement of the device, it’s noteworthy that solely relying on this property might not guarantee a durable bond, especially as the hydrogel experiences dehydration. However, this temperature-responsive adhesiveness can play a complementary role in enhancing the device’s grasp by counterbalancing any potential decrease in force due to hydrogel reswelling.
A soft robotic thera-gripper for epicardial sensing and electrical stimulating
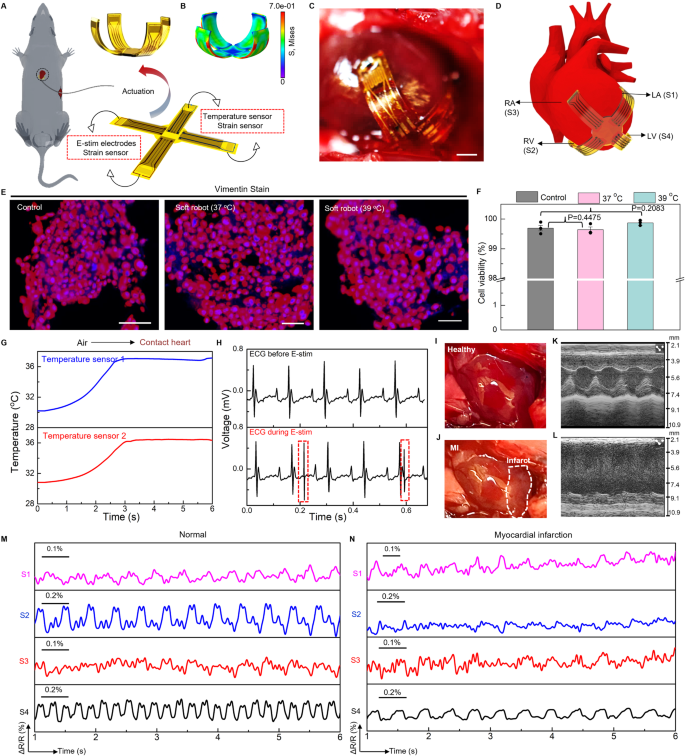
Cardiac implants that can monitor and regulate heart rhythms are critical for patients with severe cardiac diseases113,114. Despite the broad implementation of cardiac implants or pacemakers in clinical settings, however, existing devices are usually rigid, undeformable, and lack structural reconfigurability to adapt to dynamic motions of beating heart, which precludes optimum performance chronically and safely115. Here, we report a soft robotic thera-gripper that can gently envelop a heart to perform spatiotemporal monitoring of electrophysiological activity, temperature, and strain, and provide therapeutic capabilities (e.g., electrical stimulating). The soft robotic thera-gripper contains four multi-functional arms, emulating a starfish, to ensure effective latching onto the epicardial surface of which the soft mechanics ensures negligible disruption to cardiac dynamics. Fig. S41A presents an exploded view of the robotic thera-gripper, which contains actuators based on PNIPAM actuation hydrogel, two temperature sensors made of thermal resistors, two electrical stimulating electrodes made of Au, and four strain sensors made of serpentine Au/PI resistors (The fabrication approach appears in Materials and Methods, and Fig. S41B.). Figure 6A shows the thera-gripper at its resting state features minimally invasive insertion with a medical catheter, and forms a bowl shape at the actuation state, via a slightly raised temperature, to gently hold a beating heart for a safe, stable interface (Fig. 6C, D). The design employed PNIPAM hydrogel with a LCST 34 °C that is closely aligned with natural body temperature to achieve necessary shape deformation. The initial heating serves primarily to accelerate the actuation, but after achieving the desired state, continuous electrical heating becomes unnecessary. This feature allows the device to effectively adapt and function within the physiological temperature range without the need for ongoing thermal input. Figure 6B shows the corresponding FEA result of an actuated soft robotic thera-gripper. Moreover, we examined the biocompatibility of the soft robots in vitro and in vivo. Here, mouse 3T3-J-2 cells exposed to complete soft robotic devices with constituent materials including pure PNIPAM hydrogel and functional nanocomposites (e.g., AgNWs, RGO, PEDOT:PSS & MXene), remain robust healthy and maintain stable viability (Fig. 6E, F, Figs. S42, S43)116,117. Additionally, histological analysis reveals that the soft robots implanted inside chest chamber of mice induce no observable inflammation or other adverse effects to surrounding tissues, indicating good biocompatibility for long-term operation (Figs. S44, 45). The incorporated temperature sensors provide feedback information on device temperature that govern the structural transformation upon contact with cardiac tissues (Fig. 6G). The E-stim electrodes integrated into our thera-gripper can generate electrical impulses to regulate cardiac functionality (Fig. 6H and Fig. S41C, D). Fig. S46 shows measured ECG traces, highlighting effective transmission of electrical impulses (voltage ranges from 500 mV to 2 V with 1 ms width at 2.65 Hz) onto cardiac tissues. The e-skin layer consists of microelectrodes for capturing electrical activity of the heart, which serves as essential guidance in operating electrical stimulation (Fig. S47). Figs. S48, S49 showcase the simultaneous sensing and stimulation capabilities on a beating heart with an in vivo mouse model, demonstrating its capability in a broad spectrum of potential therapeutic applications118,119.
A Schematic illustration showing the thera-gripper features minimally invasive insertion at the resting state and wraps onto the surface of a beating heart at the actuation state. The thera-gripper contains four strain sensors made of serpentine Au/PI resistors, two E-stim electrodes based on Au, and two temperature sensors made of thermal resistors. B Finite element modeling of the actuation state. The colors in the legend indicate the magnitude of the von Mises stress. C Image of a soft robotic thera-gripper grasping on the epicardial surface of a living mouse heart. Scale bar, 5 mm. D Schematic illustration showing the thera-gripper position on a mouse heart, where the strain sensors (labeled as S1, S2, S3, and S4) are located onto different heart chambers for locally monitoring of dysfunctional tissue. E Confocal microscope images of 3T3-J-2 cells before (control) and after exposure to as-prepared soft robots integrated with an e-skin layer and a PNIPAM hydrogel-muscle layer, incubated at 37 °C and 39 °C for 48 h. Scale bars, 50 μm. F Comparative cell viability before and after soft robot’s exposure, indicating that 3T3-J-2 cells exposed with the soft robot and cultured at the elevated temperature (39 °C) have no decreased viability. Scale bars, 50 μm. Mean ± S.D. n = 3. P value by Unpaired t-test. G Temperature measurements from the thera-gripper during its deployment onto the mouse heart, demonstrate the device’s capability to monitor thermal variations in real-time. H The surface ECG trace during electrical stimulation using a pair of Au E-stim electrodes. Optical images of a healthy heart (I) and an injured heart 2 weeks after myocardial infarction (MI) (J). The MI area is shown by the white dashed circle in (J). M-mode echocardiographic images from a healthy (K) and post-MI heart (L). Representative measurements of local cardiac contractions before (M) and after MI (N) using a soft robotic thera-gripper wrapping onto a living mouse heart.
Cardiovascular disease is one of the leading causes of death worldwide affecting more than 17.9 million people per year120. Real-time and continuous monitoring of myocardial functions (e.g., contractility) is desired for patients with high risks of cardiac arrest, which not only ensures proactive treatment prior to occurrence of adverse events, but also provides comprehensive evaluations of therapeutic effects during postoperative care121,122,123. A soft thera-gripper, integrated with strain sensors distributed on its arms, can provide continuous, spatially resolved quantification of myocardial strains, holding great potential in precision treatment for cardiac diseases. Here, a chronic model of myocardial infarction (MI) with permanent ligation of the left coronary artery (LCA) of a mouse enables assessment of the sensing capability of a thera-gripper post to its robotic motions to establish optimized sensing interfaces (details appear in Materials and Methods.). Figure 6I, J shows the infarcted area that turns into pale myocardium. Echocardiographic strain imaging provides continuous quantification of regional myocardial function. Fig. S50A show the short-axis views in echocardiographic imaging (B-mode) from a normal and post-MI left ventricle (LV) of heart, respectively. The M-mode images, as shown in Fig. 6K, L, display the fraction shortening (FS) decreases from 75% to 15% upon occurrence of MI, indicating the heart exhibiting moderate dysfunction accompanied by regional contractility loss (Supplementary Note S7 and Fig. S50B)124,125. Although echocardiography is an invaluable tool allowing for reliable diagnostic information necessary for the clinical decision, it often lacks convenient accessibility and usually takes more than 30 min to capture the movement and function of the heart muscle and heart valves126. Figure 6D and Fig. S51A show that the multi-strain sensors (labeled as S1, S2, S3, and S4) of the implanted thera-gripper distributed across four chambers of the heart, respectively, highlighting the key advantage of our thera-gripper with real-time strain sensing over traditional imaging tools in recording local contractions continuously and simultaneously. Fig. S51B shows the response of a representative strain sensor, revealing mechanical rhythms of the cardiac cycles, consistent with ECG recordings. Ligation of coronary artery leads to myocardial infarction that causes ST-segment elevation, as shown in Fig. S51C. Figure 6M, N shows the contractility patterns of the right and left atria (RA and LA), and the right and left ventricles (RV and LV) under normal and MI conditions, respectively, measured by an implanted thera-gripper where the output features of the sensors are determined by their experienced strain that significantly correlates to their positions on epicardial surface. The S4 (LV) displays the largest amplitude due to a maximum experienced strain, indicating the intrinsic highest myocardium strength in the LV chamber. Figure 6J reveals the area of infarction 2 weeks after the MI surgery. The results in Fig. 6N demonstrate that the LV outflow obstruction changes patterns of ventricular contraction. The sensors S2 (RV) and S4 (LV) experience a reduced strain change due to the consequent loss of contractile myocardium, which, correspondingly, reduces force of myocardial contractility and decreases heart rate. Collectively, such bio-inspired design establishes a foundation for implantable robots to harness on-demand motion for structural adaptation inside body and sensory functions for real-time optimization of therapeutic outcomes.
Our post-implantation evaluation revealed that the hydrogel-based thera-gripper remained intact and securely attached to cardiac tissue as intended, demonstrating its durability and effectiveness over time (Fig. S52A). Notably, the E-stim electrodes and thermal sensor maintained optimal performance, effectively delivering electrical stimulation (Fig. S52B, C) and precisely sensing temperature fluctuations (Fig. S52D) even after a 2-week period. These results support its feasibility for long-term therapeutic and diagnostic applications.