Boston Scientific has positive modular CRM data
Boston Scientific
(NYSE: BSX)
today shared data supporting the use of its modular cardiac rhythm management (mCRM) technology.
Marlborough, Massachusetts-based Boston Scientific presented data in an abstract at Heart Rhythm Society in Boston today.
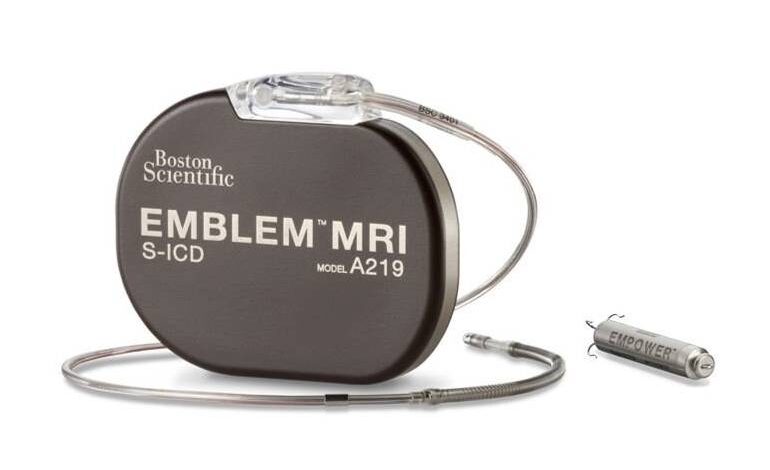
The mCRM system features a novel leadless pacemaker (LP) with wireless communication to a subcutaneous implantable cardioverter defibrillator (S-ICD). This combination delivers anti-tachycardia pacing (ATP) and bradycardia pacing to patients while avoiding transvenous lead complications.
Boston Scientific’s mCRM offering utilizes the Emblem MRI S-ICD system and the Empower modular pacing system (MPS). The company previously touted it as the first leadless pacemaker capable of delivering both bradycardia pacing support and ATP. It kicked off the MODULAR-ATP study evaluating the technology in late 2021.
The multi-center, international trial intends to enroll up to 300 patients at risk of sudden cardiac death (SCD). It evaluates LP safety and mCRM system efficacy with endpoints reported out to six months.
For safety, the trial has a primary endpoint of freedom from major LP-related complications, according to the abstract. Efficacy endpoints evaluated the wireless device communication success between the LP and S-ICD and the LP fixation mechanism. This is measured by low and stable pacing capture thresholds (PCTs).
Boston Scientific reported a major complication-free rate of 97.5%, exceeding the 86% performance criterion. Wireless communication success came in at 98.8% of the patients, exceeding the goal of 88%. The company reported that 97.4% of patients had PCTs at or below the study’s benchmark at six months, topping the 80% performance criterion. Boston Scientific said eight (4.9%) deaths occurred, but none registered an arrhythmic or procedure-related cause.
MODULAR-ATP continues to follow patients out to two years.