Global Stockpile of Cholera Vaccine Is Gone as Outbreaks Spread
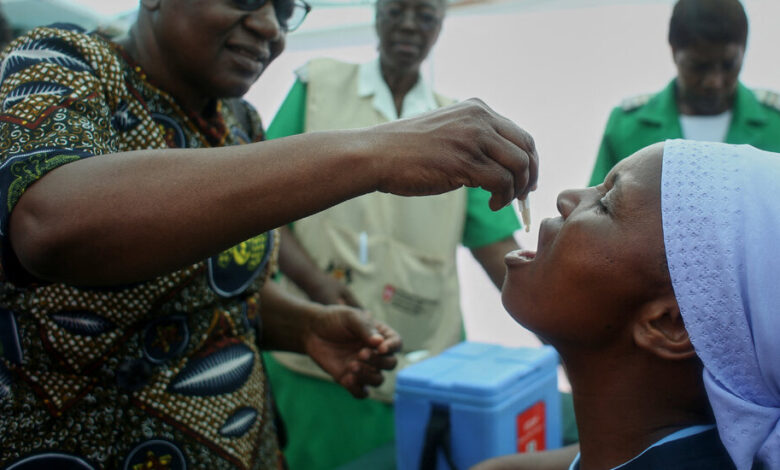
Doses of cholera vaccine are being given to patients as fast as they are produced and the global stockpile has run completely dry, as deadly outbreaks of the disease continue to spread.
This does not shock anyone in the field of emergency epidemic response because the vaccine stockpile has been precariously low for years.
The surprise — the good news, which is in itself surprising since ‘cholera’ and ‘good news’ are rarely used together — is that three new vaccine makers are setting up production lines and joining the effort to replenish the stockpile.
And a fourth company, the only one that currently makes the vaccine, which is given orally, has been working at a pace that experts describe as “heroic” to expand its production.
Yet even with all this, the total global supply of the vaccine that will become available this year will be, at best, a quarter of what is needed.
At the end of February, countries had already reported 79,300 cases and 1,100 deaths from cholera this year. Since there is no uniform system for counting cases, this is most likely a gross underestimate.
In October 2022, the organization that manages the global emergency cholera vaccine stockpile made an unprecedented recommendation that people receive only one dose of the vaccine instead of two in an effort to stretch the supply. A single dose of the cholera vaccine provides between six months and two years of immunity, while the full regimen of two doses delivered a month apart gives adults roughly four years of protection.
Last year, countries sent requests for 76 million doses of the vaccine for single-dose “reactive campaigns” — efforts to vaccinate people in places with active outbreaks.
There were only 38 million doses in the stockpile, so only half the requests were filled, and those were with only a single dose. No vaccines were left for preventive campaigns that would ideally be carried out in places such as Gaza, where all of the conditions for large outbreaks exist, or in places where cholera is endemic.
The race to make more cholera vaccine illustrates all of the reasons it’s so hard to respond to epidemics even with the participation of committed drug makers who are not scared off by the slim profit margins in an immunization that’s mostly for poor people.
Cholera can cause death by dehydration in as little as a single day as the body tries to expel virulent bacteria in streams of vomit and watery diarrhea. The disease is spread through unclean drinking water. The current outbreaks are being driven by the spread of conflict and climate disasters that force people into crowded living situations without adequate sanitation systems. In recent months, there have been outbreaks in 17 countries, including Afghanistan, Zambia and Syria.
Yet demand has only grown since then.
The South Korean company EuBiologics is currently the sole company worldwide that makes the cholera vaccine. The company had been aware for some time that there would be pressure on the supply of the vaccine because the only other firm that made it, an Indian subsidiary of the drug company Sanofi, had announced in 2018 that it would end production of the vaccine, which it did in 2023.
To cover the gap in vaccine production, Rachel Park, the director of international business at EuBiologics, said the company decided to try to simplify its vaccine formula, streamlining steps and ingredients so it could make more doses faster.
The company was then making more of the bulk drug product than it could put into tubes quickly, so it contracted a second Korean firm to assist.
EuBiologics also invested in construction of a second manufacturing site that would double the amount of the vaccine the company could make. The company has taken the lengthy and expensive steps of having both the simplified vaccine and its new facility approved by the World Health Organization in a process called prequalification, which means that countries will not have to administer their own regulatory assessments. When the new plant starts producing the company will be able to make up to 46 million doses a year.
“EuBiologics is really the unsung hero of the story,” said Dr. Julia Lynch, the director of the cholera vaccine program for the International Vaccine Institute, a United Nations-backed organization based in Seoul. “They are doing everything they can to get volumes up as fast as possible.”
Together, these steps should increase production to a total of about 46 million doses this year, and to about 90 million doses in 2025 and onward, Ms. Park said. But that will still most likely be significantly less than what the world requires.
“Doses are being allotted before they are even produced,” said Dr. Daniela Garone, the international medical coordinator for Doctors Without Borders who sits on the committee that decides which countries will receive doses, and how many. “We weren’t expecting it to be better this year, but we didn’t think it would be this much worse.”
There is some more hope on the distant horizon: Three more drug companies have cholera vaccines in their pipeline. The International Vaccine Institute has licensed its vaccine to Biological E, an Indian firm, and is sharing the formula and equipment for making it.. If all goes well, that vaccine could come to market by the end of 2026 because Biological E is a large company that already makes many products prequalified by the W.H.O.
In South Africa, a company called Biovac will soon start clinical trials on what eventually could be the first vaccine ever produced from start to finish in sub-Saharan Africa. Biovac hopes to conclude the trials by 2027. After that, it will most likely take at least a year for the vaccine to obtain W.H.O. prequalification, said Dr. Morena Makhoana, the chief executive of Biovac.
Bharat Biotech, another big Indian company with large production capacity, is working on its own oral cholera vaccine. It could bring its vaccine to market by the end of 2025.
To spur companies to invest in producing cholera vaccines, Gavi, the international organization that supplies immunizations to low- and middle-income countries, has indicated the possibility of advance market commitments — the promise of future orders that would encourage drugmakers to invest in producing the cholera vaccine. Gavi pays EuBiologics $1.53 per dose for the vaccine.
Bharat and Biological E both plan to produce about 15 million doses per year initially, Dr. Lynch said — “modest quantities” by the standards of these huge Indian companies that could make more if the market continues to grow.
The potential demand is difficult to predict, she said. “That’s really the question: Is what the world is going through right now a kind of phenomena of a few years triggered by something?” Dr. Lynch said. “Or is this a new normal? Is this a new kind of set point?”