BioTech
Insmed lung disease drug hits target in key Phase 3 trial
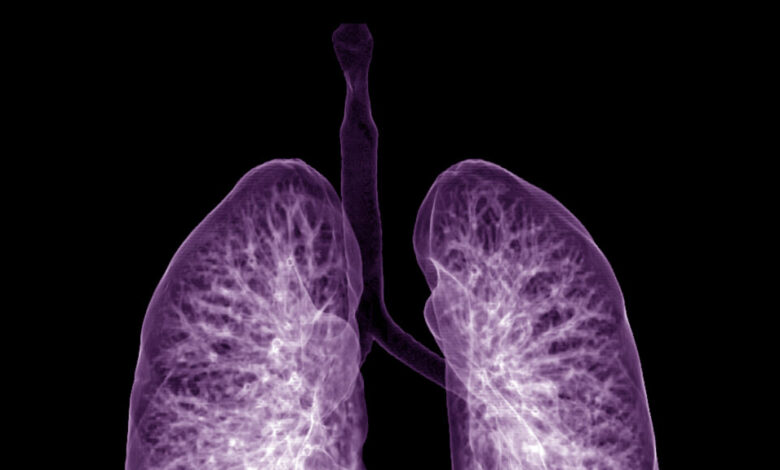
An experimental drug from Insmed Incorporated successfully reduced lung problems among patients with an airway disease in a closely watched Phase 3 trial, sending the company’s share price soaring early Tuesday.
The drug, brensocatib, reduced so-called pulmonary exacerbations by roughly 20% versus placebo in patients with bronchiectasis, hitting the trial’s primary endpoint. The trial, called the ASPEN study, tested two dosages of the drug, and the company said both significantly cut rates of pulmonary exacerbations.
Insmed shares more than doubled in pre-market trading. Analysts have forecasted that brensocatib could lead to billions of dollars in annual sales if approved.