Korea’s Koh Young to launch brain surgery robot in US in 2025
Koh Young Technology Inc., a South Korean developer of 3D optical inspection devices used in electronics manufacturing, aims to launch a brain surgery robot in the US in the first half of next year to make inroads into the world’s largest medical robot market.
Koh Young plans to apply for premarket approval for the neurosurgical robot Kymero to the US Food and Drug Administration (FDA) next week, according to industry sources on Wednesday.
The company listed on South Korea’s tech-heavy Kosdaq is expected to introduce the robot, probably with a different name, in the US in the first half of 2025 as the FDA spends a half year on a review at least.
“The US has about 1,400 hospitals with neurosurgery that can perform brain surgeries,” said a Koh Young official. “We aim to make a foray into the global market based on recognition by major South Korean hospitals.”
The global robot market is expected to more than double to $12.7 billion with North America accounting for 62% by 2025 from $5.9 billion in 2020, according to the Korea Institute of Science and Technology Information.
PERFORMS MORE THAN 500 SURGERIES
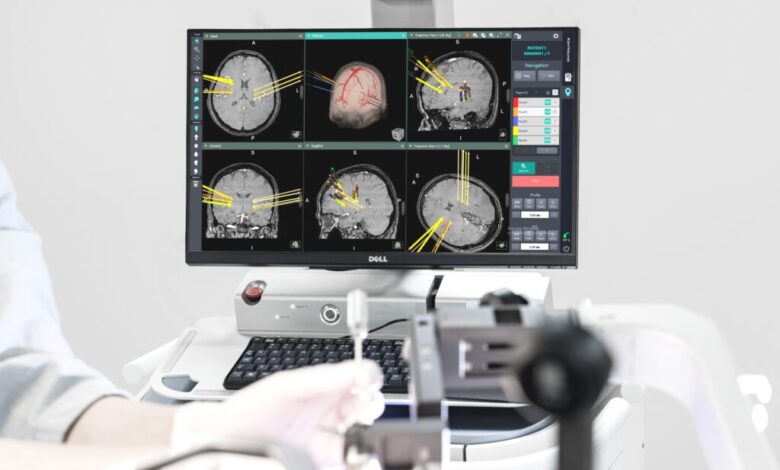
The Kymero is engineered to provide exceptional accuracy for complex stereotactic and navigated procedures, Koh Young said.
It has the world’s first optical sensor attached to a bed, which allows operators to track the robot’s positions and postures in real time. The feature reduces time for surgeries and patient sequelae.
The artificial intelligence-powered robot uses a high-precision optical sensor to navigate the patient’s brain, without physically touching nerves and arteries. It can also implant electrodes deep in the brain to treat Parkinson’s disease.
“The Kymero is excellent in terms of software when implementing images such as CT and MRI,” said Cho Chul bum, a neurosurgery professor at the Catholic University of Korea’s College of Medicine in Seoul, referring to computed tomography and magnetic resonance imaging.
FROM SEMICONDUCTOR INSPECTION SYSTEMS TO MEDICAL ROBOTS
Koh Young is the world’s No. 1 maker of 3D solder paste inspection (SPI) systems, which test printed circuit boards used for electronics, semiconductors and others to make sure that they are printed correctly.
The company has been developing medical robots since 2011 to diversify its business portfolios. Its robots have been performing more than 500 surgeries at six major local hospitals including the country’s top Seoul National University Hospital since 2020.
The firm is also developing a digital X-ray device to be linked with the neurosurgical robot with an aim to expand its product portfolios to other devices for brain surgeries starting from the device’s commercialization.
GLOBAL EXPANSION
Koh Young has been working on preliminary reviews for FDA approval with US agents since last year. It also established dedicated divisions including sales forces for the global medical robot business.
Koh Young CEO Koh Kwang-il has been focusing on the entry into the US market, leading the company’s US unit.
“Medical robots are a new growth driver after semiconductor inspection devices,” Koh told The Korea Economic Daily in an interview last year.
It will be a key to Koh Young’s future growth whether the FDA will approve the Kymero.
The company plans to do its utmost to expand the business worldwide by making inroads into other countries in Asia and Europe once the FDA approves it.
Write to Hyung-Chang Choi at calling@hankyung.com
Jongwoo Cheon edited this article.