Levels of autonomy in FDA-cleared surgical robots: a systematic review
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines22.
Data collection
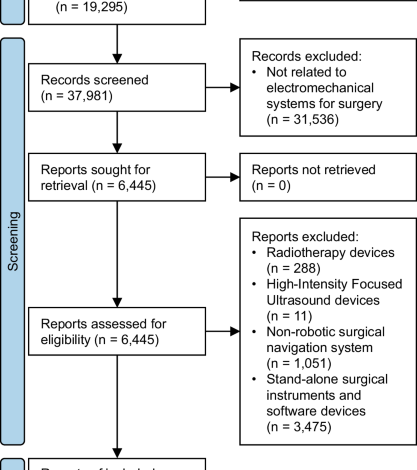
All records from the FDA 510(k) and De Novo databases with a decision date since January 1, 2015 were downloaded. Duplicate records by 510(k) Number and De Novo Number were automatically removed. AccessGUDID (Global Unique Device Identification Database) records were also collected using the online portal system with search query ((robot*) AND (surg*)) OR ((robot*) AND (intervention*)). No restriction on time was applied to the AccessGUDID search, as it was not a filter option. Duplicate AccessGUDID records by Public Device Record Key were automatically removed. Record collection for all databases occurred on March 2, 2023 and on December 11, 2023 using the same search method.
Eligibility criteria and screening process
One researcher (AL) independently and manually reviewed the database fields of all records and performed duplicate checking of all records on separate days. We excluded records with Device Classification Names (510(k) and De Novo), and Global Medical Device Nomenclature Terms (AccessGUDID) that were not directly related to electromechanical systems used for surgery. Examples of names and terms that we deemed unrelated were “polymer patient examination glove”, “wheelchair, powered”, and “general surgical procedure kit”. Examples of names and terms that we deemed related included “system, surgical, computer-controlled instrument”, “robotic surgical arm system”, and “robotic surgical navigation system”.
Reports—the full-text summary and statement documents accompanying submissions to the FDA, or device-specific pages on AccessGUDID—were retrieved and manually assessed by one researcher (AL) alongside manufacturer and distributor websites to identify eligible studies or surgical robots. We defined “surgical robot” according to the International Organization for Standardization (ISO) and International Electrotechnical Commission (IEC) definitions in ISO 8373, IEC 80601-2-77, and IEC 60601-4-1 as medical electrical equipment with a degree of autonomy that incorporates a computer-controlled electromechanical component intended to actuate, position, orient, or manipulate a surgical instrument—an invasive device with an applied part that could administer energy or invade into the patient’s body through an incision on the skin or inner surface of a natural orifice8. By this definition, we excluded robotic radiation therapy systems and robotic high-intensity focused ultrasound systems which do not require incisions, and surgical navigation systems that lack a computer-controlled electromechanical component. We also excluded stand-alone surgical instruments and software devices that were not intended for use with a surgical robot. Conversely, we included surgical instruments, software, and accessories that were intended for use with a surgical robot.
Since the FDA requires a new 510(k) submission for changes or modifications to existing devices and AccessGUDID documents all versions or models of devices, there were multiple reports corresponding to each unique surgical robot. One review author (AL) manually assessed all eligible reports alongside corresponding manufacturer and distributor websites to identify unique devices (Supplementary Methods). Older-generation surgical robots from the same company were grouped with their newest versions.
Levels of Autonomy in Surgical Robotics (LASR) taxonomy
In concordance with prior research and standardization efforts, we defined a Levels of Autonomy in Surgical Robotics (LASR) scale that clarifies the division of roles between surgeons and robotic systems during surgery2,3,5,8,9,18,23,24. Specifically, we used concepts from the framework on levels of autonomy for medical robotics originally proposed by Yang et al.18 and further tailored to surgical robotics by Fosch-Villaronga et al.23., Haidegger et al.5,24, and Attanasio et al.2. based on the emerging ISO and IEC standards3,8,9. Building upon these, we introduced additional clarifications informed by surgeon feedback to define the division of roles between surgeons and robots during surgery and human-robot interactions. Thus, the LASR taxonomy classifies each surgical robot by its highest level of autonomy capabilities from 0 (No Autonomy) to 5 (Full Autonomy) (Fig. 2).
Level 0—no autonomy
Devices without robotic equipment. The surgeon generates, selects, executes, and monitors all surgical actions, and the device provides no aid in such actions. Surgeries performed with these devices are considered identical to non-robotic manual cases.
By our definition of a “surgical robot”, we excluded Level 0 devices in this review.
Level 1—robot assistance
Surgical robots that require the surgeon to control all movements of the system and activation of its surgical instruments directly and continuously. The surgeon generates, selects, executes, and monitors all surgical actions, and the surgical robot aids the surgeon in the execution and monitoring of such actions either with passive support or active guidance. In passive support, the surgeon maintains a free range of motion while the surgical robot provides minor assistance that does not grossly interfere with the surgeon’s intended motion trajectories. Examples include teleoperation, tremor filtration, and tool tracking. In active guidance, the surgical robot provides mechanical support such as haptic feedback or motion constraints to influence the surgeon’s physical actions.
Level 2—task autonomy
Surgical robots that can execute and monitor preprogrammed, automated actions for a specific task when selected by the surgeon, without requiring the surgeon’s continuous direct control over the movements and instrument activations. The surgical robot is unable to independently define parameters to generate plans, so the surgeon needs to provide the information required to perform the action. These actions are predictable and designed to reduce variability across procedures. The preprogrammed actions may automate either a few discrete surgical gestures—the smallest meaningful interaction of a surgical instrument with human tissue—or the complete task, which involves a coordinated sequence of multiple surgical gestures25,26.
Level 3—conditional autonomy
Surgical robots that can propose various patient-specific strategies for surgical tasks or procedures that the surgeon may select from or revise, and then automatically execute and monitor the actions of the surgeon-approved plan. The robotic system extracts parameters from uploaded data streams such as preoperative patient scans to autonomously generate potential strategies for a task, and constantly monitors the surgical environment via methods like real-time intraoperative imaging to update the strategies.
Level 4—high-level autonomy
Surgical robots that can generate and proactively select the optimal patient-specific surgical plan and autonomously execute and monitor the plan upon surgeon approval. These robotic systems constantly monitor the surgical environment and autonomously make minor updates to the procedural plan as needed. If extreme changes occur intraoperatively such that the surgical robot’s uncertainty exceeds the limits for guaranteed safety, the robotic system may request the surgeon to safely intervene through methods such as temporarily handing over control to the surgeon or requesting additional inputs. Of note, the surgeon is only required to approve the plan and supervise the procedure; although the surgeon has the option to intervene when they see fit or when requested, the robotic system shall be able to complete the procedure even without surgeon intervention.
Level 5—full autonomy
Surgical robots that can independently make decisions regarding the whole surgical procedure, including preoperative workflows. These systems can generate and select the optimal patient-specific surgical plan without prior surgeon approval, and autonomously execute and monitor the plan. Although the surgeon has the option to safely intervene, these robotic systems shall be able to independently handle all environmental and adverse conditions without requesting or needing surgeon intervention.
The surgeon may supervise the procedure at any level.