Selective emergence of photoluminescence at telecommunication wavelengths from cyclic perfluoroalkylated carbon nanotubes
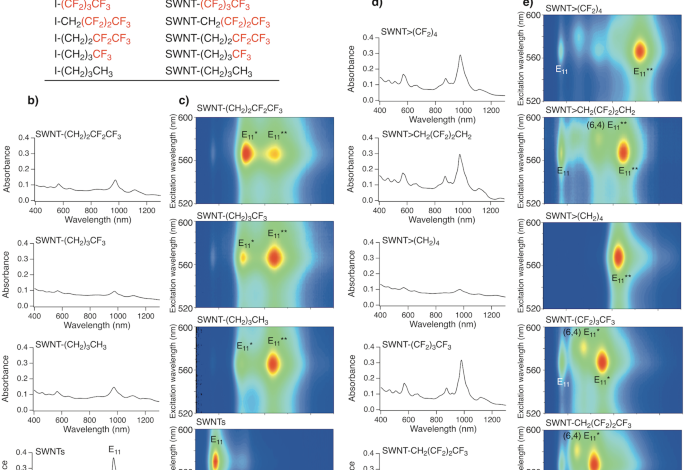
Effect of fluorine atoms on the functionalisation degree of functionalised (6,5) SWNTs
SWNTs were functionalised using 1,4-diiodooctafluorobutane (I(CF2)4I) and sodium naphthalenide. High functionalisation efficiency was expected owing to the six-membered ring structure of the resulting cycloadducts29. To clarify the effects of the two reactive sites and number of fluorine atoms, 1-iodobutane derivatives (RI, R = (CH2)3CH3, (CH2)3CF3, (CH2)2CF2CF3, CH2(CF2)2CF3, (CF2)3CF3) and 1,4-diiodo-2,2,3,3-tetrafluorobutane (ICH2(CF2)2CH2I) were used instead of I(CF2)4I; hereafter, the functionalised SWNTs are designated as SWNT-R, SWNT>CH2(CF2)2CH2, and SWNT>(CF2)4, respectively. Figure 1 and Supplementary Figs. 1, 2 show the absorption spectra and Raman spectra of the functionalised SWNTs at an excitation wavelength of 561 nm. An increase in the D band (~1300 cm−1) toward the G band (~1590 cm−1) and a decrease in the characteristic absorption peaks, which are characteristic features of sidewall functionalisation, were observed30 (Supplementary Data 1). The addition of fluoroalkyl groups was also supported by the detection of fluorine atoms using X-ray photoelectron spectroscopy (Supplementary Fig. 3). Interestingly, the D/G values after functionalisation increased in the order: [SWNT>CH2(CF2)2CH2] < [SWNT-CH2(CF2)2CF3] < [SWNT-(CF2)3CF3] < [SWNT>(CF2)4] < [SWNT-(CH2)2CF2CF3] < [SWNT-(CH2)3CF3] < [SWNT-(CH2)3CH3] < [SWNT>(CH2)4]22,29. With the exception of SWNT-CH2(CF2)2CF3 and SWNT>CH2(CF2)2CH2, the D/G values after functionalisation tended to decrease with an increasing number of fluorine atoms. These results indicate that the substitution of hydrogen atoms close to the reactive site with fluorine atoms suppressed the addition reaction of the SWNTs. Additionally, Br(CH2)4Br and I(CF2)4I, with two reactive sites, exhibited higher reactivity than CH3(CH2)3I and CF3(CF2)3I. Regarding the effect of halogen atoms at the reactive sites, the D/G values of SWNT-(CH2)3CH3 synthesised using 1-iodobutane and 1-bromobutane were 0.21 and 0.19, respectively, indicating that iodoalkane is more reactive than the corresponding bromoalkane.
a Chemical formulae of the reagents and abbreviations of the corresponding SWNT adducts. b, d Absorption spectra and c, e PL mapping of SWNTs and functionalised SWNTs with increasing numbers of fluorine substituents dispersed in D2O containing 1 wt% SDBS. The peak assignment for (6,5) SWNT (E22 and E11 absorption, E11, E11*, and E11** PL) is shown unless otherwise stated.
Effect of fluorine atoms on the PL characteristics of functionalised (6,5) SWNTs
Figure 1c depicts the PL spectra of functionalised SWNTs dispersed in a 1 wt% sodium dodecylbenzenesulfonate (SDBS) D2O solution, in which the excitation wavelengths are suitable for the excitation of (6,5) SWNTs. There was a good correlation between the changes in the E11 absorption peak intensity and the E11 PL peak intensity (Supplementary Fig. 4). SWNT-(CH2)3CF3 (1104 and 1240 nm) and SWNT-(CH2)2CF2CF3 (1118 and 1243 nm) exhibited two new PL peaks (namely, E11* and E11** PL), which are similar to SWNT-(CH2)3CH3 (1100 and 1231 nm)24 (Supplementary Table 1). In contrast, one prominent PL peak attributed to the functionalised (6,5) SWNTs was observed from SWNT-CH2(CF2)2CF3 (1119 nm), SWNT-(CF2)3CF3 (1152 nm), SWNT>(CH2)4 (1230 nm)22,29, SWNT>CH2(CF2)2CH2 (1246 nm), and SWNT>(CF2)4 (1318 nm). Notably, SWNT>(CF2)4 selectively emerged a new PL peak at 1318 nm. The PL wavelength of the functionalised (6,5) SWNTs was significantly longer than that of the corresponding alkylated SWNTs, SWNT>(CH2)422,29.
The small PL peaks observed at 1043 nm in SWNT-CH2(CF2)2CF3, 1073 nm in SWNT-(CF2)3CF3, and 1137 nm in SWNT>CH2(CF2)2CH2 were assigned to the functionalised (6,4) SWNTs, because the wavelength maxima of their excitation spectra matched the E11 and E22 absorption wavelength of (6,4) SWNTs (Supplementary Figs. 5 and 6)6. The number of PL peaks in (6,5) SWNTs emerged by functionalisation strongly depended on the number of fluorine atoms and reactive sites in the reagent. It is noteworthy that, owing to their high selectivity, CF3(CF2)2CH2I, CF3(CF2)3I, Br(CH2)4Br29, ICH2(CF2)2CH2I, and I(CF2)4I produced a single E11* or E11** PL peak, whereas the other reagents produced two PL peaks. The high selectivity of the PL wavelength suggested that a regioselective reaction occurred. Increasing the number of fluorine atoms shifted both the E11* and E11** PL wavelengths to longer wavelengths, similar to that of previously reported results20. The gradual shift of the E11* and E11** PL wavelengths, depending on the number of fluorine atoms instead of the difference in the binding sites, results in a PL wavelength change.
Effect of fluorine atoms on the PL characteristics of functionalised SWNTs with different chiral angles
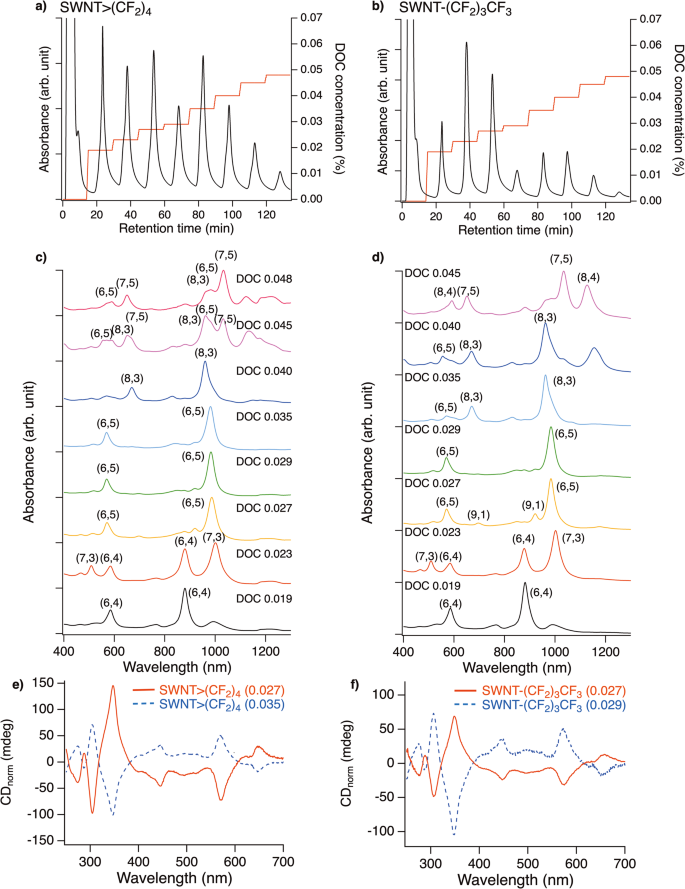
The PL contour plots of SWNT>(CF2)4 exhibited new PL peaks at 1052 and 1219 nm with an excitation wavelength of 869 nm, suggesting that I(CF2)4I is effective for significantly decreasing the local band gap of (6,4) SWNTs (Supplementary Fig. 6)12. To clarify the effect of controlling the PL properties on SWNTs other than (6,5) SWNTs, gel chromatography was conducted on SWNT>(CF2)4, SWNT>CH2(CF2)2CH2, and SWNT-(CF2)3CF3. The functionalised SWNTs were dispersed in 0.5 wt% sodium dodecylsulfate (SDS) and 0.5 wt% sodium cholate (SC) aqueous solutions and the dispersion was injected into sephacryl gel. The adsorbed SWNTs on the gel were separated using gradient high-performance liquid chromatography (HPLC) using three surfactant mixed systems developed by ref. 31. The chiral index of the SWNTs, including each fraction that was eluted depending on the sodium deoxycholate (DOC) concentration, was assigned using the absorption spectra (Fig. 2 and Supplementary Figs. 7–9). The solvent of the fraction was then exchanged with the 1 wt% SC D2O solution to measure the PL. Hereafter, the separated SWNTs are designated as SWNT-R with the DOC concentration in parentheses. The separated SWNT>(CF2)4, SWNT>CH2(CF2)2CH2, and SWNT-(CF2)3CF3 were assigned to the characteristic E11 and E22 absorption peaks. For example, separated SWNT>(CF2)4 exhibited the characteristic absorption peaks assigned to (6,4), (7,3), (9,1), (6,5), (8,3), and (7,5) SWNTs, depending on the DOC concentration, respectively (Fig. 2b). In particular, the purity of the (6,4), (6,5), and (8,3) SWNTs adducts was high because of the lack of characteristic absorption peaks assigned to the other SWNTs. The trend of the chiral index of the eluted SWNTs adducts at each DOC concentration was in good agreement with those reported in previous studies20,29,31,32. The small influence of the addenda on the separation behaviour may be due to the low functionalisation degree of SWNT>(CF2)4, SWNT>CH2(CF2)2CH2, and SWNT-(CF2)3CF3. Characteristic absorption peaks assigned to (6,5) SWNTs were observed for SWNT>(CF2)4 (0.027), SWNT>(CF2)4 (0.035), SWNT-(CF2)3CF3 (0.027), and SWNT-(CF2)3CF3 (0.029). The radial breathing mode peaks of the separated fractions also support the assignment of the chiral index of the SWNTs adducts (Supplementary Fig. 10). The circular dichroism (CD) spectra of these fractions exhibited mirror-image CD spectra similar to those of (11,−5) and (6,5) SWNTs, indicating the achievement of optical resolution for the functionalised (6,5) SWNTs (Fig. 2c).
HPLC profile of (a) SWNT>(CF2)4 and (b) SWNT-(CF2)3CF3 monitored at 280 nm and gradient proportion of DOC concentration (wt%). Condition: column, ϕ10 × 200 mm; eluent, H2O containing 0.5 wt% SC, 1.0 wt% SDS, and X wt% DOC, where X corresponds to the values shown on the gradient; flow rate, 2 mL min−1. Absorption spectra of separated c SWNT>(CF2)4 and d SWNT-(CF2)3CF3 in D2O containing 1 wt% SC. CD spectra of separated e SWNT>(CF2)4 and f SWNT-(CF2)3CF3 (right) in D2O containing 1 wt% SC normalised by the E22 absorbance.
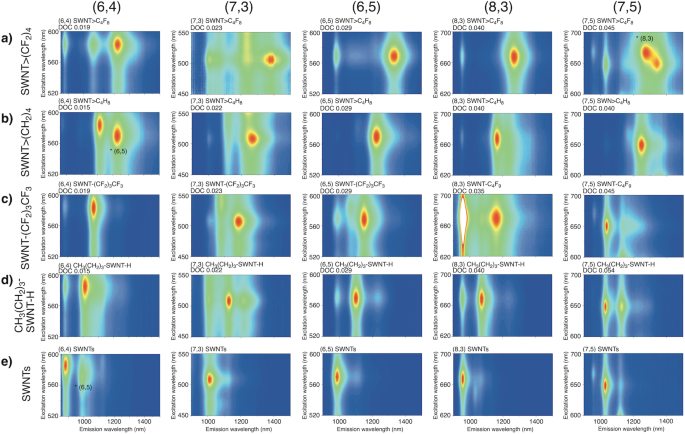
Figure 3 shows the PL contour map for each separated functionalised SWNT. One predominant PL peak emerged after the functionalisation of each separated SWNT>(CF2)4, SWNT>CH2(CF2)2CH2, SWNT>(CH2)429, and SWNT-(CF2)3CF3 (Supplementary Figs. 11–18 and Supplementary Table 2). As an exception, (6,4) SWNT>(CF2)4 (0.019) and (7,3) SWNT>(CF2)4 (0.023) exhibited two PL peaks ((6,4) SWNT: 1064 and 1222 nm and (7,3) SWNT: 1373 and 1389 nm). Interestingly, two PL peaks emerged in (6,4) and (7,3) SWNT>(CF2)4; however, the chirality dependence on the selectivity is currently unclear. Unlike functionalisation with bromobutane, which results in two new PL peaks, it is noteworthy that functionalisation using CF3(CF2)3I exhibited high selectivity to emerge a single new PL peak. The PL peaks of SWNT-(CF2)3CF3 were observed at longer wavelengths than those of the corresponding SWNT-(CH2)3CH3 regardless of the chiral index, and the PL wavelength of SWNT-(CF2)3CF3 was similar to that of the SWNT-(CF2)5CF3 reported by Wang et al. (Table 1)21. The new PL peak of SWNT>(CF2)4 was observed at the longest wavelength among those of SWNT>(CH2)429, -(CF2)3CF3, and -(CH2)3CH320, regardless of the chiral index. The results demonstrate the effectiveness of functionalisation using fluoroalkanes with two reactive sites to control the PL of SWNTs in terms of selectivity and significant wavelength shifting.
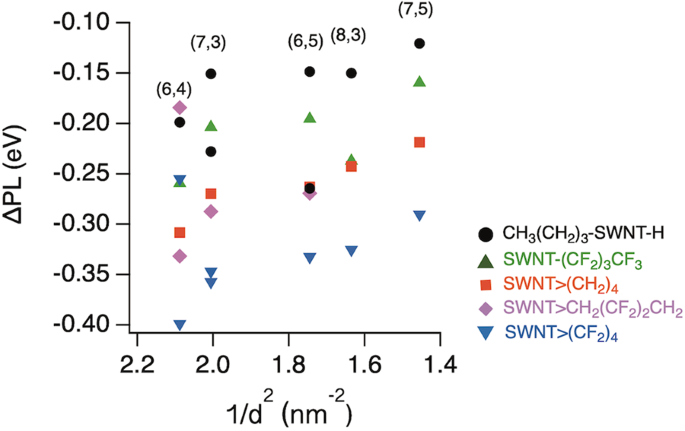
Wang et al. reported that there is a linear relationship between the PL energy difference before and after arylation and the diameter multiplier17. Figure 4 shows the PL energy differences before and after functionalisation (ΔPL) plotted as a function of the reciprocal diameter (1/d2) of the functionalised SWNTs. The ΔPL tended to increase with an increasing 1/d2 for each functionalisation, indicating that functionalisation of SWNTs with a smaller diameter is more influential in the local band gap modulation. The difference in ΔE between SWNT-(CH2)3CH3 and SWNT-(CF2)3CF3 indicates the substituent effect of the fluorine atoms. In contrast, the difference in ΔE between SWNT-(CH2)3CH3 and SWNT>(CH2)4 indicates the effect of the number of reactive sites. Therefore, the similar trends in the difference in ΔE between SWNT-(CF2)3CF3 and SWNT>(CF2)4 and between SWNT-(CH2)3CH3 and SWNT>(CH2)4 indicate that the significant PL wavelength shift in SWNT>(CF2)4 is caused by the combined effects of cyclisation using a reagent with two reactive sites and the electronic effect of the fluorine atoms. Interestingly, the ΔE of (8,3) SWNT-(CF2)3CF3 exhibited different trends from the others. The deviation from the trends of other SWNTs was also observed in the (8,3) SWNT-(CF2)5CF3 reported by ref. 21.
Mechanistic studies on controlling the functionalisation degree and PL characteristics
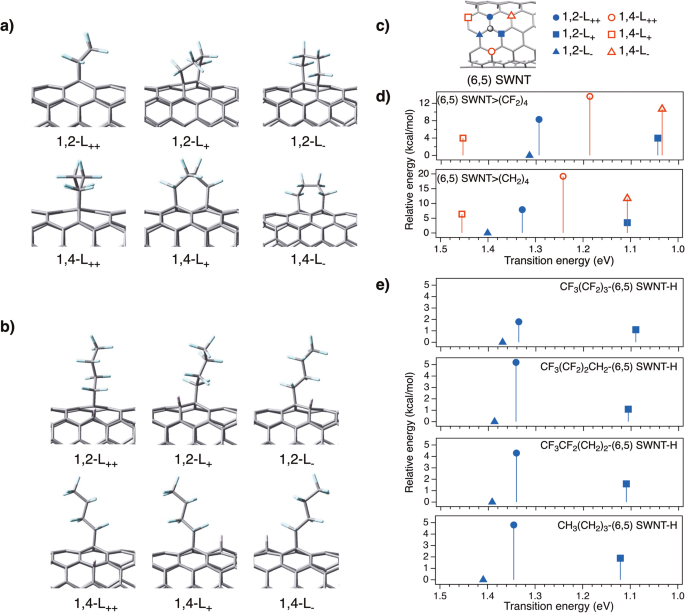
To elucidate the origin of the PL selectivity and significant PL wavelength shifts induced by fluorinated alkyl functionalisation, DFT and TD-DFT calculations were performed systematically (Fig. 5). Previous studies20,22,26,29 have demonstrated that the relative energy of the functionalised SWNTs and the spin density of the intermediates, which depends on the synthesis route and reagents of the functionalised SWNTs, are relevant factors for the binding configuration. Therefore, these factors, namely, spin density of intermediates, relative stability, and transition energy, of SWNT adducts were focused on in this study.
Optimised partial structures of a (6,5) SWNT>(CF2)4 and b CF3(CF2)3-(6,5) SWNT-H. c Six binding configurations of (6,5) SWNTs. The binding sites relative to the carbon atoms highlighted in the grey circle are presented as 1,2-L++ ( ), 1,2-L+ (
), 1,2-L+ ( ), 1,2–L– (
), 1,2–L– ( ), 1,4–L++ (
), 1,4–L++ ( ), 1,4-L+ (
), 1,4-L+ ( ), and 1,4-L– (
), and 1,4-L– ( ). d, e Calculated transition energies (eV) and relative energies (kcal/mol) of the model molecules of (6,5) SWNT>(CF2)4, (6,5) SWNT>(CH2)4, CF3(CF2)3-(6,5) SWNT-H, CF3(CF2)2CH2-(6,5) SWNT-H, CF3CF2(CH2)2-(6,5) SWNT-H, and CH3(CH2)3-(6,5) SWNT-H.
). d, e Calculated transition energies (eV) and relative energies (kcal/mol) of the model molecules of (6,5) SWNT>(CF2)4, (6,5) SWNT>(CH2)4, CF3(CF2)3-(6,5) SWNT-H, CF3(CF2)2CH2-(6,5) SWNT-H, CF3CF2(CH2)2-(6,5) SWNT-H, and CH3(CH2)3-(6,5) SWNT-H.
First, to clarify the low functionalisation degree of SWNT adducts using iodofluoroalkanes, as compared to that using 1-iodobutane, the spin density of the fluoroalkyl radicals and monofunctionalised SWNTs radicals were calculated using the UB3LYP method33,34,35,36. The six configurations of the two carbon atoms in the same hexagonal ring on the SWNTs (Fig. 5c) were classified according to their orientations with respect to the SWNT axis, denoted as L++, L+, and L–23. As shown in Table 2, the spin densities of monofunctionalised SWNT radicals on SWNTs tended to decrease as the number of fluorine atoms increased. The spin densities of 1,2-L++ and 1,2-L–, followed by 1,4-L+ were higher than those of the other carbon atoms. The spin density of the carbon atom in the CF3(CF2)3 radical (0.7880) was lower than that in the CF3(CF2)n(CH2)3-n radicals (n = 2 (1.0756), 1 (1.1109), and 0 (1.1104)) and CH3(CH2)3 radical (1.1054)) (Supplementary Table 3). These low spin densities of the carbon atom at the CF3(CF2)3 radicals and the delocalised spin density on monofunctionalised SWNTs are likely responsible for the low functionalisation degree of SWNTs adducts using iodofluoroalkanes.
Next, the transition energy and relative stability (ΔE) of the model compounds of the functionalised SWNTs (Fig. 5) were examined and the results are listed in Table 3. The partial structures of the model compounds are shown in Supplementary Figs. 19–25. The calculated transition energies showed that the binding configuration is the main factor governing the local band gap of the functionalised SWNTs. Additionally, these transition energies decreased as the number of fluorine atoms in the alkyl groups increased, regardless of the binding configuration, supporting the experimental results where the PL wavelength shifted to longer wavelengths with an increasing number of fluorine atoms.
Focusing on the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels, the lower LUMO level of (6,5) SWNT>(CF2)4 than that of (6,5) SWNT>(CH2)4 is a typical effect of the electron-withdrawing groups (Supplementary Figs. 26–28 and Supplementary Table 4). In contrast, the orbital shape or distribution of the HOMO and LUMO does not show significant difference between these two adducts (Supplementary Fig. 29). Figure 5e shows a gradual shift in the transition energy depending on the number of fluorine atoms, which is in good agreement with the PL observations. The transition energy and relative stability for the (6,4), (7,3), (8,3), and (7,5) SWNTs were also examined, and the results are summarised in Supplementary Fig. 30 and Supplementary Tables 5–7. Most of the SWNTs adducts, except for the L+ (6,4) and (8,3) SWNT adducts, exhibited a similar trend to that of the (6,5) SWNTs and agreed well with the experimental measurements; that is, the substitution of hydrogen atoms with fluorine atoms was effective in decreasing the local band gap.
A comparison of the relative stability tendency between the positional isomers of each functionalised SWNT showed that the substituted fluorine atoms influenced the relative stability, but the order of the binding configurations was similar to that of the corresponding alkylated SWNTs. The relative stability of the 1,2-adducts of (6,5) SWNT-[(CF2)3CF3]2 was significantly lower than that of the corresponding 1,4-adducts. The energy difference between the 1,2- and 1,4-adducts, as compared to that between the corresponding alkylated SWNTs, (6,5) SWNT-[(CH2)3CH3]2, was very large, suggesting a larger steric hindrance between the perfluoroalkyl groups. In addition to the low spin density of the intermediates, it is possible that steric hindrance suppressed bis-perfluoroalkylation, resulting in selective hydroperfluoroalkylation and the emergence of a single emission from SWNT-(CF2)3CF3 at 1152 nm.
As mentioned above, the spin density of plausible intermediates and the relative stability of functionalised SWNTs exhibited similar trends in the corresponding perfluoroalkylation and alkylation reactions, suggesting that the binding configurations of functionalised SWNTs using SWNTs-R are the same. Consequently, the theoretical calculation results indicate that the origin of the significantly longer wavelength-shifted PL peak in SWNT>(CF2)4 is due to the substituent effect of the fluorine atom and the molecular design of the reactant with two reaction sites. Previous studies proposed that SWNT-(CH2)3CH3 may obtain the thermodynamic product20, that is, the 1,2-addition product (1,2-L– for mod(n–m,3) = 1, 1,2-L+ for mod(n–m,3) = 2), whereas SWNT>(CH2)4 may result in the kinetic product, that is, the 1,2-L++-addition product29. These findings suggest that SWNT-(CF2)3CF3 prefers the 1,2-L− for mod(n–m,3) = 1 and the 1,2-L+ for mod(n–m,3) = 2, while SWNT>(CF2)4 is more likely to produce the 1,2-L++ adducts, respectively. Accordingly, the calculated transition energies of these adducts exhibit a trend similar to that observed in the PL wavelength shifts. The effect of the tether length of two binding sites in reactive alkylation using 1,n-dibromoalkane (Br(CH2)nBr has been reported previously, and the results indicate that the alkylation reagents having the appropriate tether length (n = 3–5) induce a new single PL peak in SWNTs29. It is known that the small ring-forming reaction is stereoelectronically favourable and has high reaction efficiency. The higher PL selectivity observed in the reaction using I(CF2)4I and ICH2(CF2)4CH2I is due to their suitable tether lengths resulting in higher efficiency of the cycloaddition reaction.
In conclusion, SWNTs were functionalised using 1-iodofluorobutane derivatives, and the results revealed that the number of fluorine atoms was effective in controlling the degree of functionalisation and selectivity of the binding configuration. Additionally, the PL peak selectivity emerged at significantly longer wavelengths by the functionalisation of I(CF2)4I, as compared with the corresponding alkylation. The PL wavelength of (6,5) SWNT>(CF2)4 was observed at 1320 nm, which is suitable for telecommunication applications. The results revealed that (6,5) SWNTs can selectively produce new NIR PL in the range of 1100–1320 nm, which is largely red-shifted from the pristine PL wavelength at 970 nm, by modifying the number of reactive sites and fluorine atoms in the alkylation reagents. In addition to the optical resolution of (11,−5) and (6,5) SWNTs, highly pure (6,4) and (8,3) SWNT>(CF2)4 were successfully obtained using gel chromatography. The PL wavelengths of (6,4), (7,3), (8,3), and (7,5) SWNT>(CF2)4 and SWNT-(CF2)3CF3 were observed at longer wavelengths than those of their corresponding SWNT>(CH2)429 and SWNT-(CH2)3CH3 adducts20. These results indicate the effectiveness of the functionalisation using perfluoroalkanes with one or two reactive sites for the large band gap modulation. The PL wavelength differences before and after functionalisation exhibited a diameter dependence. The new findings in this study increase our understanding of the functionalised SWNT structure and methods for controlling the local band gap, which will contribute to the optical application of SWNTs, such as in NIR light-emitting materials.



![DVIDS – Images – Centam Guardian Phase II: Blackhawk inserts Costa Rican telecommunications repairmen [Image 4 of 5] DVIDS – Images – Centam Guardian Phase II: Blackhawk inserts Costa Rican telecommunications repairmen [Image 4 of 5]](https://europeantech.news/wp-content/uploads/2024/06/1718771502_860_1000w_q95-390x220.jpg)