Vitestro’s robotic blood drawing device meets trial endpoints
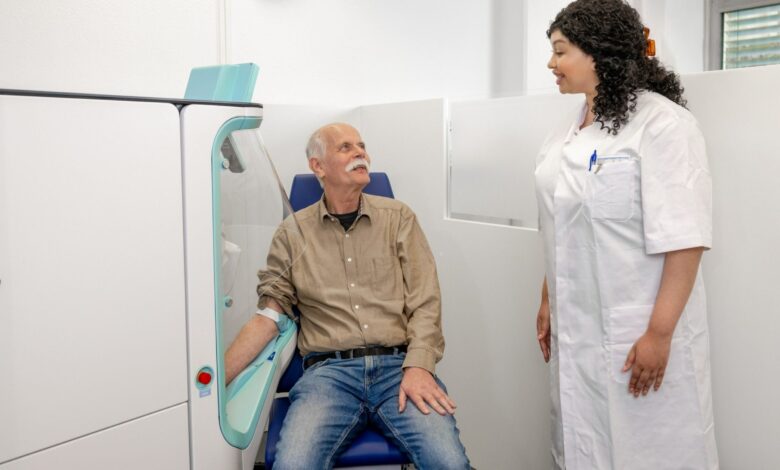
Netherland-based Vitestro has announced positive results from a trial evaluating the safety and efficacy of its autonomous blood-drawing device.
The results were from the pivotal phase of the Autonomous Blood Drawing Optimization and Performance Testing (ADOPT) study (NCT05878483). Results from the trial are expected to support approval of the device in Europe by the end of this year.
Vitestro’s device was designed using AI, advanced imaging technology and robotics. It aims to provide accurate and autonomous blood draws, which reduce the need for manual handling. It may help ease nursing workload and help alleviate staffing shortages.
The trial enrolled 609 participants in the Phase B1 and C1-1 portions of the trial, with the B1 Phase designed to demonstrate the non-inferiority and support CE-marking and Phase C1-1 being the first follow-up study after the CE-marking study was completed.
The Vitestro device met the study’s primary endpoint by achieving 95% first-stick success, comparable to manual venipuncture first-stick success rates of 93-97%, per the company. The device had a haemolysis rate of 0.6%, under the ≤2% haemolysis benchmark for the best sample collection practice defined by the American Society of Clinical Pathology. Haemolysis can occur due to inappropriate sample collection and render blood samples unable to be analysed.
The device had a median procedure time of one minute 49 seconds, with manual phlebotomy reporting a service time of five minutes including time for accessing the test order and time required for specimen draw. There were no reported serious or moderate adverse events. Mild adverse events occurred in 1.3% of the participants.
Access the most comprehensive Company Profiles
on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Company Profile – free
sample
Your download email will arrive shortly
We are confident about the
unique
quality of our Company Profiles. However, we want you to make the most
beneficial
decision for your business, so we offer a free sample that you can download by
submitting the below form
By GlobalData
The device also reported positive patient-reported outcomes, with 98% accepting the automated blood draw device. Furthermore, 83% of patients rated the procedure pain as less than or comparable to manual blood draws.
The robotics industry is expected to be worth over $218bn by 2030, per a GlobalData report. In the healthcare sector, robotics is mostly used in the surgical setting. Recently, these surgical robots have used 5G for remote robotic surgery.
Last month, Vitestro raised $22m to commercialise the robotic blood drawing device. In addition to financing European commercialisation, the funding will also support the company’s expansion into the US market.